Directed evolution of ligand dependence: small-molecule-activated protein splicing
- PMID: 15247421
- PMCID: PMC489967
- DOI: 10.1073/pnas.0402762101
Directed evolution of ligand dependence: small-molecule-activated protein splicing
Abstract
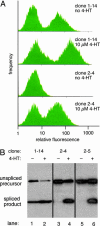
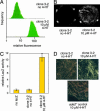
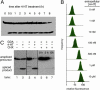
Artificial molecular switches that modulate protein activities in response to synthetic small molecules would serve as tools for exerting temporal and dose-dependent control over protein function. Self-splicing protein elements (inteins) are attractive starting points for the creation of such switches, because their insertion into a protein blocks the target protein's function until splicing occurs. Natural inteins, however, are not known to be regulated by small molecules. We evolved an intein-based molecular switch that transduces binding of a small molecule into the activation of an arbitrary protein of interest. Simple insertion of a natural ligand-binding domain into a minimal intein destroys splicing activity. To restore activity in a ligand-dependent manner, we linked protein splicing to cell survival or fluorescence in Saccharomyces cerevisiae. Iterated cycles of mutagenesis and selection yielded inteins with strong splicing activities that highly depend on 4-hydroxytamoxifen. Insertion of an evolved intein into four unrelated proteins in living cells revealed that ligand-dependent activation of protein function is general, fairly rapid, dose-dependent, and posttranslational. Our directed-evolution approach therefore evolved small-molecule dependence in a protein and also created a general tool for modulating the function of arbitrary proteins in living cells with a single cell-permeable, synthetic small molecule.
Figures

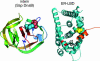
References
-
- Guo, Z., Zhou, D. & Schultz, P. G. (2000) Science 288, 2042–2045. - PubMed
-
- Lin, Q., Barbas, C. F., III, & Schultz, P. G. (2003) J. Am. Chem. Soc. 125, 612–613. - PubMed
-
- Picard, D. (2000) Methods Enzymol. 327, 385–401. - PubMed
-
- Clackson, T. (1997) Curr. Opin. Chem. Biol. 1, 210–218. - PubMed
-
- Gossen, M., Freundlieb, S., Bender, G., Muller, G., Hillen, W. & Bujard, H. (1995) Science 268, 1766–1769. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

