UVA inactivates protein tyrosine phosphatases by calpain-mediated degradation
- PMID: 15247926
- PMCID: PMC1299110
- DOI: 10.1038/sj.embor.7400190
UVA inactivates protein tyrosine phosphatases by calpain-mediated degradation
Erratum in
- EMBO Rep. 2005 Nov;6(11):1101
Abstract
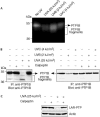
UV irradiation causes inflammatory and proliferative cellular responses. We have proposed previously that these effects are, to a large extent, caused by the ligand-independent activation of several receptor tyrosine kinases due to the inactivation of their negative control elements, the protein tyrosine phosphatases (PTPs). We examined the mechanism of this inactivation and found that, in addition to reversible oxidation of PTPs, UV triggers a novel mechanism: induced degradation of PTPs by calpain, which requires both calpain activation and substrate PTP oxidative modification. This as yet unrecognized effect of UV is irreversible, occurs predominantly with UVA and UVB, the range of wavelengths in sunlight that reach the skin surface, and at physiologically relevant doses.
Figures
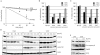
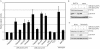


References
-
- Barrett WC, DeGnore JP, Konig S, Fales HM, Keng YF, Zhang ZY, Yim MB, Chock PB (1999) Regulation of PTP1B via glutathionylation of the active site cysteine 215. Biochemistry 38: 6699–6705 - PubMed
-
- Burridge K, Nelson A (1995) An in-gel assay for protein tyrosine phosphatase activity: detection of widespread distribution in cells and tissues. Anal Biochem 232: 56–64 - PubMed
-
- Coffer PJ, Burgering BM, Peppelenbosch MP, Bos JL, Kruijer W (1995) UV activation of receptor tyrosine kinase activity. Oncogene 11: 561–569 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

