Trends in cochlear implants
- PMID: 15247993
- PMCID: PMC4111484
- DOI: 10.1177/108471380400800102
Trends in cochlear implants
Abstract
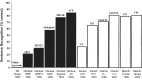
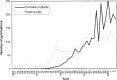
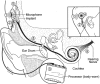
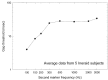
More than 60,000 people worldwide use cochlear implants as a means to restore functional hearing. Although individual performance variability is still high, an average implant user can talk on the phone in a quiet environment. Cochlear-implant research has also matured as a field, as evidenced by the exponential growth in both the patient population and scientific publication. The present report examines current issues related to audiologic, clinical, engineering, anatomic, and physiologic aspects of cochlear implants, focusing on their psychophysical, speech, music, and cognitive performance. This report also forecasts clinical and research trends related to presurgical evaluation, fitting protocols, signal processing, and postsurgical rehabilitation in cochlear implants. Finally, a future landscape in amplification is presented that requires a unique, yet complementary, contribution from hearing aids, middle ear implants, and cochlear implants to achieve a total solution to the entire spectrum of hearing loss treatment and management.
Figures














References
-
- Abbas PJ, Brown CJ. Electrically evoked brainstem potentials in cochlear implant patients with multi-electrode stimulation. Hear Res 36: 153–162, 1988 - PubMed
-
- Andreev AM, Gersuni GV, Volokhov AA. On the electrical excitability of the human ear: On the effect of alternating currents on the affected auditory apparatus. J Physiol USSR 18: 250–265, 1935
-
- Arlinger S, Gatehouse S, Bentler RA, et al. Report of the Eriksholm Workshop on auditory deprivation and acclimatization. Ear Hear 17: 87S–98S, 1996 - PubMed
-
- Bekesy GV. Experiments in Hearing. New York: McGraw-Hill, 1960
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
