Characterization of a transport and detoxification pathway for the antitumour drug bleomycin in Saccharomyces cerevisiae
- PMID: 15248838
- PMCID: PMC1134087
- DOI: 10.1042/BJ20040392
Characterization of a transport and detoxification pathway for the antitumour drug bleomycin in Saccharomyces cerevisiae
Abstract
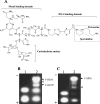
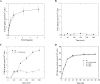
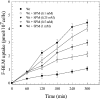
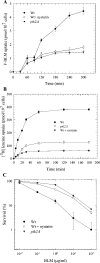
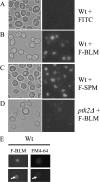
BLM (bleomycin) is effective in combination therapy against various cancers including testicular cancer. However, several other cancers such as colon cancer are refractory to BLM treatment. The exact mechanism for this differential response of cancer cells to the drug is not known. In the present study, we created fluorescently labelled BLM-A5, which retained nearly full genotoxic potential, and used this molecule to conduct the first study to understand the transport pathway of the drug in Saccharomyces cerevisiae. Uptake studies revealed that fluoro-BLM-A5 is transported into the cell in a concentration-dependent manner. Transport of a non-saturating concentration of fluoro-BLM-A5 was modest for the first 90 min, but thereafter it was sharply induced until 300 min. The inducible transport was completely abolished by the addition of cycloheximide, suggesting that BLM-A5 uptake into the cell is dependent on new protein synthesis. Interestingly, transport of fluoro-BLM-A5 was blocked if the cells were preincubated with increasing concentrations of spermine. Moreover, a mutant lacking the Ptk2 kinase, necessary for positively regulating polyamine transport, was defective in fluoro-BLM-A5 uptake and exhibited extreme resistance to the drug. A simple interpretation of these results is that BLM-A5 may enter the cell through the polyamine transport system. We showed further that after the uptake, fluoro-BLM-A5 accumulated into the vacuole of the parent, but localized to the cytoplasm of mutants disrupted for the END3 gene required for an early step of the endocytotic pathway. In general, mutants with a defect in the endocytic pathway to the vacuole were hypersensitive to BLM-A5. We suggest that BLM-A5 is transported across the yeast plasma membrane and sequestered into the vacuole for detoxification.
Figures



Similar articles
-
The human carnitine transporter SLC22A16 mediates high affinity uptake of the anticancer polyamine analogue bleomycin-A5.J Biol Chem. 2010 Feb 26;285(9):6275-84. doi: 10.1074/jbc.M109.046151. Epub 2009 Dec 25. J Biol Chem. 2010. PMID: 20037140 Free PMC article.
-
A genome-wide screen in Saccharomyces cerevisiae reveals altered transport as a mechanism of resistance to the anticancer drug bleomycin.Cancer Res. 2004 Feb 1;64(3):1102-9. doi: 10.1158/0008-5472.can-03-2729. Cancer Res. 2004. PMID: 14871844
-
The Yeast Permease Agp2 Senses Cycloheximide and Undergoes Degradation That Requires the Small Protein Brp1-Cellular Fate of Agp2 in Response to Cycloheximide.Int J Mol Sci. 2023 Apr 10;24(8):6975. doi: 10.3390/ijms24086975. Int J Mol Sci. 2023. PMID: 37108141 Free PMC article.
-
A new twist in cellular resistance to the anticancer drug bleomycin-A5.Curr Drug Metab. 2010 Sep;11(7):595-602. doi: 10.2174/138920010792927307. Curr Drug Metab. 2010. PMID: 20812903 Review.
-
[Derivative of bleomycin generates less of ROS? Less of fibrosis? Alternative in the development of an efficacy anticancer therapy but less toxic].Bull Cancer. 2010 May;97(5):527-34. doi: 10.1684/bdc.2009.1034. Bull Cancer. 2010. PMID: 20100681 Review. French.
Cited by
-
The human carnitine transporter SLC22A16 mediates high affinity uptake of the anticancer polyamine analogue bleomycin-A5.J Biol Chem. 2010 Feb 26;285(9):6275-84. doi: 10.1074/jbc.M109.046151. Epub 2009 Dec 25. J Biol Chem. 2010. PMID: 20037140 Free PMC article.
-
Histatin 5 uptake by Candida albicans utilizes polyamine transporters Dur3 and Dur31 proteins.J Biol Chem. 2011 Dec 23;286(51):43748-43758. doi: 10.1074/jbc.M111.311175. Epub 2011 Oct 27. J Biol Chem. 2011. PMID: 22033918 Free PMC article.
-
Loss of the yeast transporter Agp2 upregulates the pleiotropic drug-resistant pump Pdr5 and confers resistance to the protein synthesis inhibitor cycloheximide.PLoS One. 2024 May 22;19(5):e0303747. doi: 10.1371/journal.pone.0303747. eCollection 2024. PLoS One. 2024. PMID: 38776347 Free PMC article.
-
Suppression of sensitivity to drugs and antibiotics by high external cation concentrations in fission yeast.PLoS One. 2015 Mar 20;10(3):e0119297. doi: 10.1371/journal.pone.0119297. eCollection 2015. PLoS One. 2015. PMID: 25793410 Free PMC article.
-
Role of the plasma membrane transporter of organic cations OCT1 and its genetic variants in modern liver pharmacology.Biomed Res Int. 2013;2013:692071. doi: 10.1155/2013/692071. Epub 2013 Jul 31. Biomed Res Int. 2013. PMID: 23984399 Free PMC article. Review.
References
-
- Hecht S. M. Bleomycin: new perspectives on the mechanism of action. J. Nat. Prod. 2000;63:158–168. - PubMed
-
- Ramotar D., Wang H. Protective mechanisms against the antitumor agent bleomycin: lessons from Saccharomyces cerevisiae. Curr. Genet. 2003;43:213–224. - PubMed
-
- Wharam M. D., Phillips T. L., Kane L., Utley J. F. Response of a murine solid tumor to in vivo combined chemotherapy and irradiation. Radiology. 1973;109:451–455. - PubMed
-
- Umezawa H. Natural and artificial bleomycins: chemistry and antitumor activities. Pure Appl. Chem. 1971;28:665–680. - PubMed
-
- Jani J. P., Mistry J. S., Morris G., Davies P., Lazo J. S., Sebti S. M. In vivo circumvention of human colon carcinoma resistance to bleomycin. Cancer Res. 1992;52:2931–2937. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

