Drs2p-coupled aminophospholipid translocase activity in yeast Golgi membranes and relationship to in vivo function
- PMID: 15249668
- PMCID: PMC489982
- DOI: 10.1073/pnas.0404146101
Drs2p-coupled aminophospholipid translocase activity in yeast Golgi membranes and relationship to in vivo function
Abstract
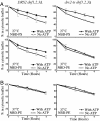
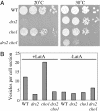
Aminophospholipid translocases (APLTs) are defined primarily by their ability to flip fluorescent or spin-labeled derivatives of phosphatidylserine (PS) and phosphatidylethanolamine (PE) from the external leaflet of a membrane bilayer to the cytosolic leaflet and are thought to establish phospholipid asymmetry in biological membranes. The identities of APLTs remain unknown, although candidate proteins include the Drs2p/ATPase II subfamily of P-type ATPases. Drs2p from budding yeast localizes to the trans-Golgi network (TGN), and here we show that this membrane contains an ATP-dependent APLT that flips 7-nitro-2-1,3-benzoxadiazol-4-yl (NBD) PS and PE derivatives from the luminal to the cytosolic leaflet. To assess the contribution of Drs2p to this activity, TGN membranes were prepared from strains harboring WT or temperature-sensitive alleles of DRS2 and null alleles of three other potential APLT genes (DNF1, DNF2, and DNF3). Assay of these membranes indicated that Drs2p was required for the ATP-dependent translocation of NBD-PS, whereas no active translocation of NBD-PE or NBD-phosphatidylcholine was detected. The specificity of Drs2p for NBD-PS suggested that translocation of PS would be required for the function of Drs2p in protein transport from the TGN. However, cho1 yeast strains that are unable to synthesize PS do not phenocopy drs2 but instead transport proteins normally via the secretory pathway. In addition, a drs2 cho1 double mutant retains drs2 transport defects. Therefore, whereas NBD-PS is a preferred substrate for Drs2p in vitro, endogenous PS is not an obligatory substrate in vivo for the role Drs2p plays in protein transport.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

