The Treacher Collins syndrome (TCOF1) gene product is involved in ribosomal DNA gene transcription by interacting with upstream binding factor
- PMID: 15249688
- PMCID: PMC489999
- DOI: 10.1073/pnas.0402492101
The Treacher Collins syndrome (TCOF1) gene product is involved in ribosomal DNA gene transcription by interacting with upstream binding factor
Abstract
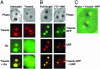
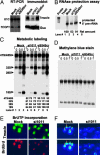
Treacher Collins syndrome (TCS) is an autosomal dominant disorder characterized by an abnormality of craniofacial development that arises during early embryogenesis. TCS is caused by mutations in the gene TCOF1, which encodes the nucleolar phosphoprotein treacle. Even though the genetic alterations causing TCS have been uncovered, the mechanism underlying its pathogenesis and the function of treacle remain unknown. Here, we show that treacle is involved in ribosomal DNA gene transcription by interacting with upstream binding factor (UBF). Immunofluorescence labeling shows treacle and UBF colocalize to specific nucleolar organizer regions and cosegregate within nucleolar caps of actinomycin d-treated HeLa cells. Biochemical analysis shows the association of treacle and UBF with chromatin. Immunoprecipitation and the yeast two-hybrid system both suggest physical interaction of the two nucleolar phosphoproteins. Down-regulation of treacle expression using specific short interfering RNA results in inhibition of ribosomal DNA transcription and cell growth. A similar correlation is observed in Tcof(+/-) mouse embryos that exhibit craniofacial defects and growth retardation. Thus, treacle haploinsufficiency in TCS patients might result in abnormal development caused by inadequate ribosomal RNA production in the prefusion neural folds during the early stages of embryogenesis. The elucidation of a physiological function of treacle provides important information of relevance to the molecular dissection of the biochemical pathology of TCS.
Figures



References
-
- Sulik, K. K., Johnston, M. C., Smiley, S. J., Speight, H. S. & Jarvis, B. E. (1987) Am. J. Med. Genet. 27, 359-372. - PubMed
-
- Jabs, E. W., Li, X., Coss, C. A., Taylor, E. W., Meyers, D. A. & Weber, J. L. (1991) Genomics 11, 193-198. - PubMed
-
- Loftus, S. K., Edwards, S. J., Scherpbier-Heddema, T., Buetow, K. H., Wasmuth, J. J. & Dixon M. J. (1993) Hum. Mol. Genet. 2, 1785-1792. - PubMed
-
- The Treacher Collins Syndrome Collaborative Group (1996) Nat. Genet. 12, 130-136. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

