Herpesviral Fcgamma receptors: culprits attenuating antiviral IgG?
- PMID: 15251110
- PMCID: PMC7173100
- DOI: 10.1016/j.intimp.2004.05.020
Herpesviral Fcgamma receptors: culprits attenuating antiviral IgG?
Abstract
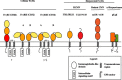
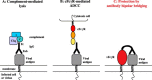
Production of IgG in response to virus infection is central to antiviral immune effector functions and a hallmark of B cell memory. Antiviral antibodies (Abs) recognising viral glycoproteins or protein antigen displayed on the surface of virions or virus-infected cells are crucial in rendering the virus noninfectious and in eliminating viruses or infected cells, either acting alone or in conjunction with complement. In many instances, passive transfer of Abs is sufficient to protect from viral infection. Herpesviruses (HV) are equipped with a large array of immunomodulatory functions which increase the efficiency of infection by dampening the antiviral immunity. Members of the alpha- and beta-subfamily of the Herpesviridae are distinct in encoding transmembrane glycoproteins which selectively bind IgG via its Fc domain. The Fc-binding proteins constitute viral Fcgamma receptors (vFcgammaRs) which are expressed on the cell surface of infected cells. Moreover, vFcgammaRs are abundantly incorporated into the envelope of virions. Despite their molecular and structural heterogeneity, the vFcgammaRs generally interfere with IgG-mediated effector functions like antibody (Ab)-dependent cellular cytolysis, complement activation and neutralisation of infectivity of virions. vFcgammaRs may thus contribute to the limited therapeutic potency of antiherpesviral IgG in clinical settings. A detailed molecular understanding of vFcgammaRs opens up the possibility to design recombinant IgG molecules resisting vFcgammaRs. Engineering IgG with a better antiviral efficiency represents a new therapeutic option against herpesviral diseases.
Figures
Similar articles
-
Identification and Functional Characterization of a Novel Fc Gamma-Binding Glycoprotein in Rhesus Cytomegalovirus.J Virol. 2019 Feb 5;93(4):e02077-18. doi: 10.1128/JVI.02077-18. Print 2019 Feb 15. J Virol. 2019. PMID: 30487278 Free PMC article.
-
Human cytomegalovirus antagonizes activation of Fcγ receptors by distinct and synergizing modes of IgG manipulation.Elife. 2021 Mar 16;10:e63877. doi: 10.7554/eLife.63877. Elife. 2021. PMID: 33724188 Free PMC article.
-
A novel assay for detecting virus-specific antibodies triggering activation of Fcγ receptors.J Immunol Methods. 2013 Jan 31;387(1-2):21-35. doi: 10.1016/j.jim.2012.09.006. Epub 2012 Sep 27. J Immunol Methods. 2013. PMID: 23023090
-
Viral interference with antibody and complement.Semin Cell Dev Biol. 1998 Jun;9(3):329-37. doi: 10.1006/scdb.1998.0242. Semin Cell Dev Biol. 1998. PMID: 9665870 Free PMC article. Review.
-
Antibody Fc-chimerism and effector functions: When IgG takes advantage of IgA.Front Immunol. 2023 Feb 2;14:1037033. doi: 10.3389/fimmu.2023.1037033. eCollection 2023. Front Immunol. 2023. PMID: 36817447 Free PMC article. Review.
Cited by
-
The Roles of Host and Viral Antibody Fc Receptors in Herpes Simplex Virus (HSV) and Human Cytomegalovirus (HCMV) Infections and Immunity.Front Immunol. 2019 Sep 6;10:2110. doi: 10.3389/fimmu.2019.02110. eCollection 2019. Front Immunol. 2019. PMID: 31555298 Free PMC article. Review.
-
Cytomegalovirus restricts ICOSL expression on antigen-presenting cells disabling T cell co-stimulation and contributing to immune evasion.Elife. 2021 Jan 18;10:e59350. doi: 10.7554/eLife.59350. Elife. 2021. PMID: 33459589 Free PMC article.
-
The herpesviral Fc receptor fcr-1 down-regulates the NKG2D ligands MULT-1 and H60.J Exp Med. 2006 Aug 7;203(8):1843-50. doi: 10.1084/jem.20060514. Epub 2006 Jul 10. J Exp Med. 2006. PMID: 16831899 Free PMC article.
-
Maternal antibodies induced by a live attenuated vaccine protect neonatal mice from cytomegalovirus.NPJ Vaccines. 2023 Feb 3;8(1):8. doi: 10.1038/s41541-023-00602-4. NPJ Vaccines. 2023. PMID: 36737485 Free PMC article.
-
Antibody-mediated protection against genital herpes simplex virus type 2 disease in mice by Fc gamma receptor-dependent and -independent mechanisms.J Reprod Immunol. 2008 Jun;78(1):58-67. doi: 10.1016/j.jri.2007.08.004. Epub 2007 Oct 24. J Reprod Immunol. 2008. PMID: 17950908 Free PMC article.
References
-
- Sissons J.G., Bain M., Wills M.R. Latency and reactivation of human cytomegalovirus. J. Infect. 2002;44:73–77. - PubMed
-
- Abendroth A., Arvin A. Varicella-zoster virus immune evasion. Immunol. Rev. 1999;168:143–156. - PubMed
-
- Hengel H., Brune W., Koszinowski U.H. Immune evasion by cytomegalovirus-survival strategies of a highly adapted opportunist. Trends Microbiol. 1998;6:190–197. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

