Hairy transcriptional repression targets and cofactor recruitment in Drosophila
- PMID: 15252443
- PMCID: PMC449821
- DOI: 10.1371/journal.pbio.0020178
Hairy transcriptional repression targets and cofactor recruitment in Drosophila
Abstract
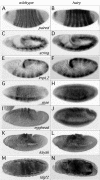
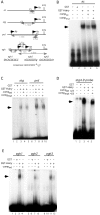
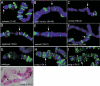
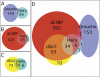
Members of the widely conserved Hairy/Enhancer of split family of basic Helix-Loop-Helix repressors are essential for proper Drosophila and vertebrate development and are misregulated in many cancers. While a major step forward in understanding the molecular mechanism(s) surrounding Hairy-mediated repression was made with the identification of Groucho, Drosophila C-terminal binding protein (dCtBP), and Drosophila silent information regulator 2 (dSir2) as Hairy transcriptional cofactors, the identity of Hairy target genes and the rules governing cofactor recruitment are relatively unknown. We have used the chromatin profiling method DamID to perform a global and systematic search for direct transcriptional targets for Drosophila Hairy and the genomic recruitment sites for three of its cofactors: Groucho, dCtBP, and dSir2. Each of the proteins was tethered to Escherichia coli DNA adenine methyltransferase, permitting methylation proximal to in vivo binding sites in both Drosophila Kc cells and early embryos. This approach identified 40 novel genomic targets for Hairy in Kc cells, as well as 155 loci recruiting Groucho, 107 loci recruiting dSir2, and wide genomic binding of dCtBP to 496 loci. We also adapted DamID profiling such that we could use tightly gated collections of embryos (2-6 h) and found 20 Hairy targets related to early embryogenesis. As expected of direct targets, all of the putative Hairy target genes tested show Hairy-dependent expression and have conserved consensus C-box-containing sequences that are directly bound by Hairy in vitro. The distribution of Hairy targets in both the Kc cell and embryo DamID experiments corresponds to Hairy binding sites in vivo on polytene chromosomes. Similarly, the distributions of loci recruiting each of Hairy's cofactors are detected as cofactor binding sites in vivo on polytene chromosomes. We have identified 59 putative transcriptional targets of Hairy. In addition to finding putative targets for Hairy in segmentation, we find groups of targets suggesting roles for Hairy in cell cycle, cell growth, and morphogenesis, processes that must be coordinately regulated with pattern formation. Examining the recruitment of Hairy's three characterized cofactors to their putative target genes revealed that cofactor recruitment is context-dependent. While Groucho is frequently considered to be the primary Hairy cofactor, we find here that it is associated with only a minority of Hairy targets. The majority of Hairy targets are associated with the presence of a combination of dCtBP and dSir2. Thus, the DamID chromatin profiling technique provides a systematic means of identifying transcriptional target genes and of obtaining a global view of cofactor recruitment requirements during development.
Conflict of interest statement
The authors have declared that no conflicts of interest exist.
Figures




References
-
- Agard DA, Hiraoka Y, Shaw P, Sedat JW. Fluorescence microscopy in three dimensions. Methods Cell Biol. 1989;30:353–377. - PubMed
-
- Andersen AS, Hansen PH, Schäffer L, Kristensen C. A new secreted insect protein belonging to the immunoglobulin superfamily binds insulin and related peptides and inhibits their activities. J Biol Chem. 2000;275:16948–16953. - PubMed
-
- Andrew DJ, Scott M. New York: Academic Press; 1994. Immunological methods for mapping protein distribution on polytene chromosomes. In: Goldstein LSB, Fyrberg, EA, editors. Drosophila melanogaster Practical uses in cell and molecular biology; pp. 353–370. - PubMed
-
- Baker DA, Mille-Baker B, Wainwright SM, Ish-Horowicz D, Dibb NJ. Mae mediates MAP kinase phosphorylation of Ets transcription factors in Drosophila . Nature. 2001;411:330–334. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

