HIV infection of naturally occurring and genetically reprogrammed human regulatory T-cells
- PMID: 15252446
- PMCID: PMC449855
- DOI: 10.1371/journal.pbio.0020198
HIV infection of naturally occurring and genetically reprogrammed human regulatory T-cells
Abstract
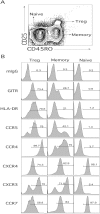
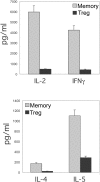
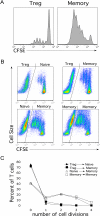
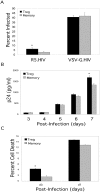
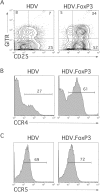
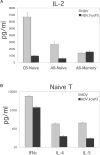
A T-cell subset, defined as CD4(+)CD25(hi) (regulatory T-cells [Treg cells]), was recently shown to suppress T-cell activation. We demonstrate that human Treg cells isolated from healthy donors express the HIV-coreceptor CCR5 and are highly susceptible to HIV infection and replication. Because Treg cells are present in very few numbers and are difficult to expand in vitro, we genetically modified conventional human T-cells to generate Treg cells in vitro by ectopic expression of FoxP3, a transcription factor associated with reprogramming T-cells into a Treg subset. Overexpression of FoxP3 in naïve human CD4(+) T-cells recapitulated the hyporesponsiveness and suppressive function of naturally occurring Treg cells. However, FoxP3 was less efficient in reprogramming memory T-cell subset into regulatory cells. In addition, FoxP3-transduced T-cells also became more susceptible to HIV infection. Remarkably, a portion of HIV-positive individuals with a low percentage of CD4(+) and higher levels of activated T-cells have greatly reduced levels of FoxP3(+)CD4(+)CD25(hi) T-cells, suggesting disruption of the Treg cells during HIV infection. Targeting and disruption of the T-cell regulatory system by HIV may contribute to hyperactivation of conventional T-cells, a characteristic of HIV disease progression. Moreover, the ability to reprogram human T-cells into Treg cells in vitro will greatly aid in decoding their mechanism of suppression, their enhanced susceptibility to HIV infection, and the unique markers expressed by this subset.
Conflict of interest statement
The authors have declared that no conflicts of interest exist.
Figures



References
-
- Annacker O, Burlen-Defranoux O, Pimenta-Araujo R, Cumano A, Bandeira A. Regulatory CD4 T cells control the size of the peripheral activated/memory CD4 T cell compartment. J Immunol. 2000;164:3573–3580. - PubMed
-
- Annacker O, Pimenta-Araujo R, Burlen-Defranoux O, Barbosa TC, Cumano A, et al. CD25+ CD4+ T cells regulate the expansion of peripheral CD4 T cells through the production of IL-10. J Immunol. 2001;166:3008–3018. - PubMed
-
- Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+CD25 high regulatory cells in human peripheral blood. J Immunol. 2001;167:1245–1253. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

