Behavioral state instability in orexin knock-out mice
- PMID: 15254084
- PMCID: PMC6729542
- DOI: 10.1523/JNEUROSCI.0586-04.2004
Behavioral state instability in orexin knock-out mice
Abstract
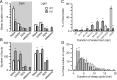
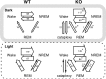
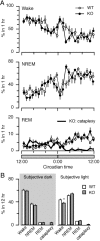
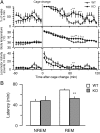
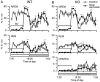
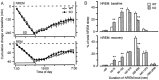
Narcolepsy is caused by a lack of orexin (hypocretin), but the physiologic process that underlies the sleepiness of narcolepsy is unknown. Using orexin knock-out (KO) mice as a model of narcolepsy, we critically tested the three leading hypotheses: poor circadian control of sleep and wakefulness, inadequate activation of arousal regions, or abnormal sleep homeostasis. Compared with wild-type (WT) littermates, orexin KO mice had essentially normal amounts of sleep and wake, but wake and non-rapid eye movement (NREM) bouts were very brief, with many more transitions between all behavioral states. In constant darkness, orexin KO mice had normal amplitude and timing of sleep-wake rhythms, providing no evidence for disordered circadian control. When placed in a new, clean cage, both groups of mice remained awake for approximately 45 min, demonstrating that, even in the absence of orexin, fundamental arousal regions can be engaged to produce sustained wakefulness. After depriving mice of sleep for 2-8 hr, orexin KO mice recovered their NREM and rapid eye movement sleep deficits at comparable rates and to the same extent as WT mice, with similar increases in EEG delta power, suggesting that their homeostatic control of sleep is normal. These experiments demonstrate that the fragmented wakefulness of orexin deficiency is not a consequence of abnormal sleep homeostasis, poor circadian control, or defective fundamental arousal systems. Instead, the fragmented behavior of orexin KO mice may be best described as behavioral state instability, with apparently low thresholds to transition between states.
Figures

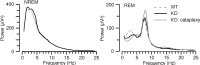
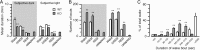
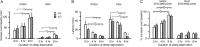
References
-
- Abrahamson EE, Leak RK, Moore RY (2001) The suprachiasmatic nucleus projects to posterior hypothalamic arousal systems. NeuroReport 12: 435-440. - PubMed
-
- Aston-Jones G, Chen S, Zhu Y, Oshinsky ML (2001) A neural circuit for circadian regulation of arousal. Nat Neurosci 4: 732-738. - PubMed
-
- Besset A, Tafti M, Nobile L, Billiard M (1994) Homeostasis and narcolepsy. Sleep 17: S29-S34. - PubMed
-
- Borbély AA, Tobler I (1996) Sleep regulation: relation to photoperiod, sleep duration, waking activity, and torpor. In: Progress in brain research (Buijs RM, Kalsbeek A, Romijn HJ, Pennartz CMA, Nirmiran M, eds), pp 343-348. Amsterdam: Elsevier. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
