Human TRPC5 channel activated by a multiplicity of signals in a single cell
- PMID: 15254149
- PMCID: PMC1665180
- DOI: 10.1113/jphysiol.2004.065391
Human TRPC5 channel activated by a multiplicity of signals in a single cell
Erratum in
- J Physiol. 2004 Nov 1;560(Pt 3):950
Abstract
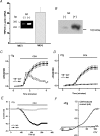
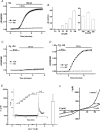
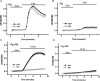
Here we explore the activation mechanisms of human TRPC5, a putative cationic channel that was cloned from a region of the X chromosome associated with mental retardation. No basal activity was evident but activity was induced by carbachol stimulation of muscarinic receptors independently of Ca2+ release. This is 'receptor activation', as described for mouse TRPC5. In addition, and in the absence of receptor stimulation, extracellular gadolinium (0.1 mm) activated TRPC5, an effect that was mimicked by 5-20 mm extracellular Ca2+ with intracellular Ca2+ buffered. We refer to this as 'external ionic activation'. TRPC5 was also activated by modest elevation of [Ca2+]i in the absence of GTP--'calcium activation'. A putative fourth activation mechanism is a signal from depleted intracellular Ca2+ stores. Consistent with this idea, human TRPC5 was activated by a standard store-depletion/Ca2+ re-entry protocol, an effect that was difficult to explain by calcium activation. Multiplicity of TRPC5 activation was demonstrated in single cells and thus not dependent on heterogeneity of expression levels or cellular context. Therefore, human TRPC5 is activated by a range of stimuli, avoiding dependence on a single critical activator as in many other ion channels. One of these stimuli would seem to be a change in Ca2+ handling by the endoplasmic reticulum.
Figures
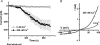

References
-
- Beech DJ, Xu SZ, McHugh D, Flemming R. TRPC1 store-operated cationic channel subunit. Cell Calcium. 2003;33:433–440. - PubMed
-
- Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. - PubMed
-
- Cui J, Bian JS, Kagan A, McDonald TV. CaT1 contributes to the stores-operated calcium current in Jurkat T-lymphocytes. J Biol Chem. 2002;277:47175–47183. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

