Dendritically released transmitters cooperate via autocrine and retrograde actions to inhibit afferent excitation in rat brain
- PMID: 15254151
- PMCID: PMC1665137
- DOI: 10.1113/jphysiol.2004.066159
Dendritically released transmitters cooperate via autocrine and retrograde actions to inhibit afferent excitation in rat brain
Abstract
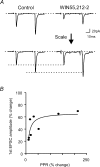
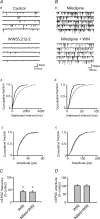
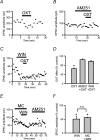
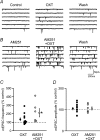
Oxytocin is released from supraoptic magnocellular neurones and is thought to act at presynaptic receptors to inhibit transmitter release. We now show that this effect is mediated by endocannabinoids, but that oxytocin nonetheless plays an important role in endocannabinoid signalling. WIN55,212-2, a cannabinoid receptor agonist, mimicked the action of oxytocin and occluded oxytocin-induced presynaptic inhibition. The cannabinoid action is at the presynaptic terminal as shown by alteration in paired pulse ratio, a reduction in miniature EPSC frequency and immunohistochemical localization of CB1 receptors on presynaptic terminals. AM251, a CB1 receptor antagonist, blocked both the WIN55,212-2 and the oxytocin-induced presynaptic inhibition of EPSCs. Depolarization of postsynaptic magnocellular neurones (which contain fatty acid amide hydrolase, a cannabinoid catabolic enzyme) caused a transient inhibition of EPSCs that could be blocked by both the AM251 and Manning compound, an oxytocin/vasopressin receptor antagonist. This indicates that somatodendritic peptide release and action on previously identified autoreceptors facilitates the release of endocannabinoids that act as mediators of presynaptic inhibition.
Figures





Similar articles
-
Endocannabinoids mediate presynaptic inhibition of glutamatergic transmission in rat ventral tegmental area dopamine neurons through activation of CB1 receptors.J Neurosci. 2004 Jan 7;24(1):53-62. doi: 10.1523/JNEUROSCI.4503-03.2004. J Neurosci. 2004. PMID: 14715937 Free PMC article.
-
Retrograde regulation of GABA transmission by the tonic release of oxytocin and endocannabinoids governs postsynaptic firing.J Neurosci. 2007 Feb 7;27(6):1325-33. doi: 10.1523/JNEUROSCI.2676-06.2007. J Neurosci. 2007. PMID: 17287507 Free PMC article.
-
Retroinhibition of presynaptic Ca2+ currents by endocannabinoids released via postsynaptic mGluR activation at a calyx synapse.J Neurosci. 2004 Jun 30;24(26):5955-65. doi: 10.1523/JNEUROSCI.0768-04.2004. J Neurosci. 2004. PMID: 15229243 Free PMC article.
-
Modulation of synaptic transmission by oxytocin and vasopressin in the supraoptic nucleus.Prog Brain Res. 2002;139:235-46. doi: 10.1016/s0079-6123(02)39020-4. Prog Brain Res. 2002. PMID: 12436939 Review.
-
Dendritic Release of Neurotransmitters.Compr Physiol. 2016 Dec 6;7(1):235-252. doi: 10.1002/cphy.c160007. Compr Physiol. 2016. PMID: 28135005 Free PMC article. Review.
Cited by
-
Opposing crosstalk between leptin and glucocorticoids rapidly modulates synaptic excitation via endocannabinoid release.J Neurosci. 2006 Jun 14;26(24):6643-50. doi: 10.1523/JNEUROSCI.5126-05.2006. J Neurosci. 2006. PMID: 16775153 Free PMC article.
-
Cell signaling dependence of rapid glucocorticoid-induced endocannabinoid synthesis in hypothalamic neuroendocrine cells.Neurobiol Stress. 2019 Mar 21;10:100158. doi: 10.1016/j.ynstr.2019.100158. eCollection 2019 Feb. Neurobiol Stress. 2019. PMID: 31193551 Free PMC article.
-
Minireview: rapid glucocorticoid signaling via membrane-associated receptors.Endocrinology. 2006 Dec;147(12):5549-56. doi: 10.1210/en.2006-0981. Epub 2006 Aug 31. Endocrinology. 2006. PMID: 16946006 Free PMC article. Review.
-
Activity-dependent regulation of synapses by retrograde messengers.Neuron. 2009 Jul 30;63(2):154-70. doi: 10.1016/j.neuron.2009.06.021. Neuron. 2009. PMID: 19640475 Free PMC article. Review.
-
Information coding in vasopressin neurons--the role of asynchronous bistable burst firing.Biosystems. 2013 May;112(2):85-93. doi: 10.1016/j.biosystems.2013.03.010. Epub 2013 Mar 14. Biosystems. 2013. PMID: 23499814 Free PMC article.
References
-
- Adan RAH, van Leeuwen FW, Sonnemans MAF, Brouns M, Hoffman G, Verbalis JG, Burbach JPH. Rat oxytocin receptor in brain, pituitary, mammary gland, and uterus: Partial sequence and immunocytochemical localization. Endocrinology. 1995;136:4022–4028. - PubMed
-
- Ames F. A clinical and metabolic study of acute intoxication with Cannabis sativa and its role in the model psychoses. J Ment Sci. 1958;104:972–999. - PubMed
-
- Barberis C, Mouillac B, Durroux T. Structural bases of vasopressin/oxytocin receptor function. J Endocrinol. 1998;156:223–229. - PubMed
-
- Biswas B, Ghosh JJ. Delta-9-tetrahydrocannabinol and lysergic acid diethylamide: comparative changes in the supraoptic and paraventricular neurosecretory activities in rat hypothalamus. Anat Anz. 1975;138:324–331. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

