Inhibition of infectious pancreatic necrosis virus replication by atlantic salmon Mx1 protein
- PMID: 15254166
- PMCID: PMC446136
- DOI: 10.1128/JVI.78.15.7938-7944.2004
Inhibition of infectious pancreatic necrosis virus replication by atlantic salmon Mx1 protein
Abstract
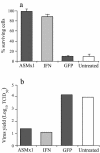
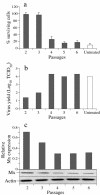
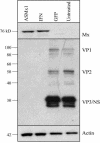
Mx proteins form a family of interferon (IFN)-induced GTPases with potent antiviral activity against various single-stranded RNA viruses in mammals and chickens. In fish, alpha/beta IFN has been reported to inhibit the replication of infectious pancreatic necrosis virus (IPNV), but the mode of action has not been elucidated. A correlation between the inhibition of IPNV and Mx protein expression has, however, been observed. To examine whether Atlantic salmon Mx1 protein (ASMx1) possesses antiviral activity against IPNV, CHSE-214 cells constitutively expressing ASMx1 were established. ASMx1 appeared to be localized in the cytoplasm. The ASMx1-expressing clone selected showed a severely reduced IPNV-induced cytopathic effect, which was confirmed by a 500-fold reduction in virus yield. The antiviral activity against IPNV was further confirmed by the inhibition of virus protein synthesis and the reduced accumulation of virus transcripts. The present work further adds to the body of evidence which suggests that antiviral activity is a major functional role of vertebrate Mx proteins. Moreover, the list of viruses inhibited by Mx proteins is extended to include double-stranded RNA viruses.
Figures


Similar articles
-
Constitutive expression of Atlantic salmon Mx1 protein in CHSE-214 cells confers resistance to infectious salmon anaemia virus.Virol J. 2005 Aug 26;2:75. doi: 10.1186/1743-422X-2-75. Virol J. 2005. PMID: 16124877 Free PMC article.
-
Effect of double-stranded RNA and interferon on the antiviral activity of Atlantic salmon cells against infectious salmon anemia virus and infectious pancreatic necrosis virus.Fish Shellfish Immunol. 2002 Sep;13(3):221-41. doi: 10.1006/fsim.2001.0397. Fish Shellfish Immunol. 2002. PMID: 12365733
-
Interferon mediated antiviral activity against salmonid fish viruses in BF-2 and other cell lines.Vet Immunol Immunopathol. 2006 Mar 15;110(1-2):1-10. doi: 10.1016/j.vetimm.2005.08.023. Epub 2005 Sep 19. Vet Immunol Immunopathol. 2006. PMID: 16169598
-
Immunization with viral antigens: infectious pancreatic necrosis.Dev Biol Stand. 1997;90:191-9. Dev Biol Stand. 1997. PMID: 9270848 Review.
-
The Mx GTPase family of interferon-induced antiviral proteins.Microbes Infect. 2007 Nov-Dec;9(14-15):1636-43. doi: 10.1016/j.micinf.2007.09.010. Epub 2007 Sep 14. Microbes Infect. 2007. PMID: 18062906 Review.
Cited by
-
Alpha interferon and not gamma interferon inhibits salmonid alphavirus subtype 3 replication in vitro.J Virol. 2010 Sep;84(17):8903-12. doi: 10.1128/JVI.00851-10. Epub 2010 Jun 23. J Virol. 2010. PMID: 20573808 Free PMC article.
-
Transcriptome profiling of antiviral immune and dietary fatty acid dependent responses of Atlantic salmon macrophage-like cells.BMC Genomics. 2017 Sep 8;18(1):706. doi: 10.1186/s12864-017-4099-2. BMC Genomics. 2017. PMID: 28886690 Free PMC article.
-
Phenotype gene expression differences between resistant and susceptible salmon families to IPNV.Fish Physiol Biochem. 2014 Jun;40(3):887-96. doi: 10.1007/s10695-013-9894-3. Epub 2013 Dec 4. Fish Physiol Biochem. 2014. PMID: 24306554
-
Host and viral determinants of Mx2 antiretroviral activity.J Virol. 2014 Jul;88(14):7738-52. doi: 10.1128/JVI.00214-14. Epub 2014 Apr 23. J Virol. 2014. PMID: 24760893 Free PMC article.
-
Constitutive expression of Atlantic salmon Mx1 protein in CHSE-214 cells confers resistance to infectious salmon anaemia virus.Virol J. 2005 Aug 26;2:75. doi: 10.1186/1743-422X-2-75. Virol J. 2005. PMID: 16124877 Free PMC article.
References
-
- Arnheiter, H., M. Frese, R. Kambadur, E. Meier, and O. Haller. 1996. Mx transgenic mice—animal models of health. Curr. Top. Microbiol Immunol. 206:119-147. - PubMed
-
- Asano, A., J. H. Ko, T. Morozumi, N. Hamashima, and T. Watanabe. 2002. Polymorphisms and the antiviral property of porcine Mx1 protein. J. Vet. Med. Sci. 64:1085-1089. - PubMed
-
- Bazzigher, L., A. Schwarz, and P. Staeheli. 1993. No enhanced influenza virus resistance of murine and avian cells expressing cloned duck Mx protein. Virology 195:100-112. - PubMed
-
- Bernasconi, D., U. Schultz, and P. Staeheli. 1995. The interferon-induced Mx protein of chickens lacks antiviral activity. J. Interferon Cytokine Res. 15:47-53. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

