Transport of African swine fever virus from assembly sites to the plasma membrane is dependent on microtubules and conventional kinesin
- PMID: 15254171
- PMCID: PMC446130
- DOI: 10.1128/JVI.78.15.7990-8001.2004
Transport of African swine fever virus from assembly sites to the plasma membrane is dependent on microtubules and conventional kinesin
Abstract
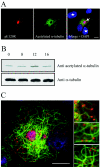
African swine fever virus (ASFV) is a large DNA virus that assembles in perinuclear viral factories located close to the microtubule organizing center. In this study, we have investigated the mechanism by which ASFV reaches the cell surface from the site of assembly. Immunofluorescence microscopy revealed that at 16 h postinfection, mature virions were aligned along microtubules. Furthermore, virus movement to the cell periphery was inhibited when microtubules were depolymerized by nocodazole. In addition, ASFV infection resulted in the increased acetylation of microtubules as well as their protection against depolymerization by nocodazole. Immunofluorescence microscopy showed that conventional kinesin was recruited to virus factories and to a large fraction of virus particles in the cytoplasm. Consistent with a role for conventional kinesin during ASFV egress to the cell periphery, overexpression of the cargo-binding domain of the kinesin light chain severely inhibited the movement of particles to the plasma membrane. Based on our observations, we propose that ASFV is recognized as cargo by conventional kinesin and uses this plus-end microtubule motor to move from perinuclear assembly sites to the plasma membrane.
Figures






Similar articles
-
Vimentin rearrangement during African swine fever virus infection involves retrograde transport along microtubules and phosphorylation of vimentin by calcium calmodulin kinase II.J Virol. 2005 Sep;79(18):11766-75. doi: 10.1128/JVI.79.18.11766-11775.2005. J Virol. 2005. PMID: 16140754 Free PMC article.
-
African swine fever virus infection disrupts centrosome assembly and function.J Gen Virol. 2005 Mar;86(Pt 3):589-594. doi: 10.1099/vir.0.80623-0. J Gen Virol. 2005. PMID: 15722518
-
African swine fever virus interaction with microtubules.Biol Cell. 1993;78(3):229-34. doi: 10.1016/0248-4900(93)90134-z. Biol Cell. 1993. PMID: 8241964
-
African swine fever virus morphogenesis.Virus Res. 2013 Apr;173(1):29-41. doi: 10.1016/j.virusres.2012.09.016. Epub 2012 Oct 8. Virus Res. 2013. PMID: 23059353 Review.
-
African swine fever virus organelle rearrangements.Virus Res. 2013 Apr;173(1):76-86. doi: 10.1016/j.virusres.2012.12.014. Epub 2013 Jan 3. Virus Res. 2013. PMID: 23291273 Review.
Cited by
-
Association of ebola virus matrix protein VP40 with microtubules.J Virol. 2005 Apr;79(8):4709-19. doi: 10.1128/JVI.79.8.4709-4719.2005. J Virol. 2005. PMID: 15795257 Free PMC article.
-
Life cycle of phytoreoviruses visualized by electron microscopy and tomography.Front Microbiol. 2013 Oct 16;4:306. doi: 10.3389/fmicb.2013.00306. eCollection 2013. Front Microbiol. 2013. PMID: 24137159 Free PMC article. Review.
-
Real-time analysis of quantum dot labeled single porcine epidemic diarrhea virus moving along the microtubules using single particle tracking.Sci Rep. 2019 Feb 4;9(1):1307. doi: 10.1038/s41598-018-37789-9. Sci Rep. 2019. PMID: 30718724 Free PMC article.
-
Amoebal Tubulin Cleavage Late during Infection Is a Characteristic Feature of Mimivirus but Not of Marseillevirus.Microbiol Spectr. 2022 Dec 21;10(6):e0275322. doi: 10.1128/spectrum.02753-22. Epub 2022 Dec 1. Microbiol Spectr. 2022. PMID: 36453900 Free PMC article.
-
Eclipse phase of herpes simplex virus type 1 infection: Efficient dynein-mediated capsid transport without the small capsid protein VP26.J Virol. 2006 Aug;80(16):8211-24. doi: 10.1128/JVI.02528-05. J Virol. 2006. PMID: 16873277 Free PMC article.
References
-
- Afonso, C. L., C. Alcaraz, A. Brun, M. D. Sussman, D. V. Onisk, J. M. Escribano, and D. L. Rock. 1992. Characterization of p30, a highly antigenic membrane and secreted protein of African swine fever virus. Virology 189:368-373. - PubMed
-
- Allan, V. J., H. M. Thompson, and M. A. McNiven. 2002. Motoring around the Golgi. Nat. Cell Biol. 4:E236-E242. - PubMed
-
- Alonso, C., J. Miskin, B. Hernaez, P. Fernandez-Zapatero, L. Soto, C. Canto, I. Rodriguez-Crespo, L. Dixon, and J. M. Escribano. 2001. African swine fever virus protein p54 interacts with the microtubular motor complex through direct binding to light-chain dynein. J. Virol. 75:9819-9827. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

