Herpes simplex virus type 1 infection induces the stabilization of p53 in a USP7- and ATM-independent manner
- PMID: 15254178
- PMCID: PMC446092
- DOI: 10.1128/JVI.78.15.8068-8077.2004
Herpes simplex virus type 1 infection induces the stabilization of p53 in a USP7- and ATM-independent manner
Abstract
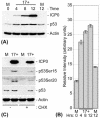
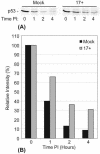
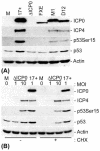
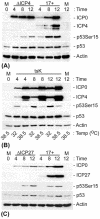
The major oncoprotein p53 regulates several cellular antiproliferation pathways that can be triggered in response to a variety of cellular stresses, including viral infection. The stabilization of p53 is a key factor in the ability of cells to initiate an efficient transcriptional response after cellular stress. Here we present data demonstrating that herpes simplex virus type 1 (HSV-1) infection of HFFF-2 cells, a low-passage-number nontransformed human primary cell line, results in the stabilization of p53. This process required viral immediate-early gene expression but occurred independently of the viral regulatory protein ICP0 and viral DNA replication. No specific viral protein could be identified as being solely responsible for the effect, which appears to be a cellular response to developing HSV-1 infections. HSV-1 infection also induced the phosphorylation of p53 at residues Ser15 and Ser20, which have previously been implicated in its stabilization in response to DNA damage. However, an HSV-1 infection of ATM(-/-) cells, which lack a kinase implicated in these phosphorylation events, did not lead to the phosphorylation of p53 at these residues, but nonetheless p53 was stabilized. We also show that the wild-type p53 expressed by osteosarcoma U2OS cells can be stabilized in response to DNA damage induced by UV irradiation, but not in response to HSV-1 infection. These data suggest that multiple cellular mechanisms are initiated to stabilize p53 during an HSV-1 infection. These mechanisms occur independently of ICP0 and its ability to sequester USP7 and may differ from those initiated in response to DNA damage.
Figures



Similar articles
-
Enhanced phosphorylation of transcription factor sp1 in response to herpes simplex virus type 1 infection is dependent on the ataxia telangiectasia-mutated protein.J Virol. 2007 Sep;81(18):9653-64. doi: 10.1128/JVI.00568-07. Epub 2007 Jul 3. J Virol. 2007. PMID: 17609267 Free PMC article.
-
Reciprocal activities between herpes simplex virus type 1 regulatory protein ICP0, a ubiquitin E3 ligase, and ubiquitin-specific protease USP7.J Virol. 2005 Oct;79(19):12342-54. doi: 10.1128/JVI.79.19.12342-12354.2005. J Virol. 2005. PMID: 16160161 Free PMC article.
-
The ATM and Rad3-Related (ATR) Protein Kinase Pathway Is Activated by Herpes Simplex Virus 1 and Required for Efficient Viral Replication.J Virol. 2018 Feb 26;92(6):e01884-17. doi: 10.1128/JVI.01884-17. Print 2018 Mar 15. J Virol. 2018. PMID: 29263259 Free PMC article.
-
Radioresistance in carcinoma of the breast.Breast. 2004 Dec;13(6):452-60. doi: 10.1016/j.breast.2004.08.004. Breast. 2004. PMID: 15563851 Review.
-
Apoptosis during herpes simplex virus infection.Adv Virus Res. 2007;69:67-97. doi: 10.1016/S0065-3527(06)69002-7. Adv Virus Res. 2007. PMID: 17222692 Review.
Cited by
-
Development of an HSV-1 neutralization test with a glycoprotein D specific antibody for measurement of neutralizing antibody titer in human sera.Virol J. 2016 Mar 18;13:44. doi: 10.1186/s12985-016-0508-4. Virol J. 2016. PMID: 26987753 Free PMC article.
-
Maintaining Genome Integrity: Protein Kinases and Phosphatases Orchestrate the Balancing Act of DNA Double-Strand Breaks Repair in Cancer.Int J Mol Sci. 2023 Jun 16;24(12):10212. doi: 10.3390/ijms241210212. Int J Mol Sci. 2023. PMID: 37373360 Free PMC article. Review.
-
Cellular players in the herpes simplex virus dependent apoptosis balancing act.Viruses. 2009 Dec;1(3):965-78. doi: 10.3390/v1030965. Epub 2009 Nov 18. Viruses. 2009. PMID: 21994577 Free PMC article.
-
Adaptive homeostasis and the p53 isoform network.EMBO Rep. 2021 Dec 6;22(12):e53085. doi: 10.15252/embr.202153085. Epub 2021 Nov 15. EMBO Rep. 2021. PMID: 34779563 Free PMC article. Review.
-
DNA-PK and ATM drive phosphorylation signatures that antagonistically regulate cytokine responses to herpesvirus infection or DNA damage.Cell Syst. 2024 Apr 17;15(4):339-361.e8. doi: 10.1016/j.cels.2024.03.003. Epub 2024 Apr 8. Cell Syst. 2024. PMID: 38593799 Free PMC article.
References
-
- Aubert, M., and J. A. Blaho. 2001. Modulation of apoptosis during herpes simplex virus infection in human cells. Microbes Infect. 3:859-866. - PubMed
-
- Banks, L., D. Pim, and M. Thomas. 2003. Viruses and the 26S proteasome: hacking into destruction. Trends Biochem. Sci. 28:452-459. - PubMed
-
- Bargonetti, J., P. N. Friedman, S. E. Kern, B. Vogelstein, and C. Prives. 1991. Wild-type but not mutant p53 immunopurified proteins bind to sequences adjacent to the SV40 origin of replication. Cell 65:1083-1091. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

