Appearance of the bona fide spiral tubule of ORF virus is dependent on an intact 10-kilodalton viral protein
- PMID: 15254180
- PMCID: PMC446139
- DOI: 10.1128/JVI.78.15.8085-8093.2004
Appearance of the bona fide spiral tubule of ORF virus is dependent on an intact 10-kilodalton viral protein
Abstract
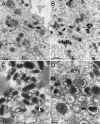
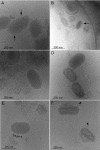
Parapoxviruses can be morphologically distinguished from other poxviruses in conventional negative staining electron microscopy (EM) by their ovoid appearance and the spiral tubule surrounding the virion's surface. However, this technique may introduce artifacts. We have examined Orf virus (ORFV; the prototype species of the Parapoxvirus genus) by cryoelectron microscopy (cryo-EM) and cryo-negative staining EM. From these studies we suggest that the shape and unique spiral tubule are authentic features of the parapoxviruses. We also constructed an ORFV mutant deleted of a gene encoding a 10-kDa protein, which is an orthologue of the vaccinia virus (VACV) 14-kDa fusion protein, and investigated its ultrastructure. This mutant virus multiplied slowly in permissive cells and produced infectious but morphologically aberrant particles. Mutant virions lacked the spiral tubule but displayed short disorganized tubules similar to those observed on the surface of VACV. In addition, thin extensions or loop-like structures were appended to the ORFV mutant particles. We suggest that these appended structures arise from a failure of the mutant virus particles to properly seal and that the sealing activity is dependent on the 10-kDa protein.
Figures



Similar articles
-
Investigation of orf virus structure and morphogenesis using recombinants expressing FLAG-tagged envelope structural proteins: evidence for wrapped virus particles and egress from infected cells.J Gen Virol. 2009 Mar;90(Pt 3):614-625. doi: 10.1099/vir.0.005488-0. J Gen Virol. 2009. PMID: 19218206
-
Poxvirus virions: their surface ultrastructure and interaction with the surface membrane of host cells.J Electron Microsc (Tokyo). 1999;48(6):937-46. doi: 10.1093/oxfordjournals.jmicro.a023768. J Electron Microsc (Tokyo). 1999. PMID: 10742959
-
A new recombinant Orf virus (ORFV, Parapoxvirus) protects rabbits against lethal infection with rabbit hemorrhagic disease virus (RHDV).Vaccine. 2011 Nov 15;29(49):9256-64. doi: 10.1016/j.vaccine.2011.09.121. Epub 2011 Oct 11. Vaccine. 2011. PMID: 22001119
-
Orf virus: A promising new therapeutic agent.Rev Med Virol. 2019 Jan;29(1):e2013. doi: 10.1002/rmv.2013. Epub 2018 Oct 28. Rev Med Virol. 2019. PMID: 30370570 Review.
-
Orf virus infection and host immunity.Curr Opin Infect Dis. 2006 Apr;19(2):127-31. doi: 10.1097/01.qco.0000216622.75326.ef. Curr Opin Infect Dis. 2006. PMID: 16514336 Review.
Cited by
-
Genomic Characterization of Orf Virus Strain D1701-V (Parapoxvirus) and Development of Novel Sites for Multiple Transgene Expression.Viruses. 2019 Jan 30;11(2):127. doi: 10.3390/v11020127. Viruses. 2019. PMID: 30704093 Free PMC article.
-
The structure of a putative scaffolding protein of immature poxvirus particles as determined by electron microscopy suggests similarity with capsid proteins of large icosahedral DNA viruses.J Virol. 2007 Oct;81(20):11075-83. doi: 10.1128/JVI.00594-07. Epub 2007 Aug 1. J Virol. 2007. PMID: 17670837 Free PMC article.
-
Insights into the evolution of a complex virus from the crystal structure of vaccinia virus D13.Structure. 2011 Jul 13;19(7):1011-20. doi: 10.1016/j.str.2011.03.023. Structure. 2011. PMID: 21742267 Free PMC article.
-
Advancing ORFV-Based Therapeutics to the Clinical Stage.Rev Med Virol. 2025 May;35(3):e70038. doi: 10.1002/rmv.70038. Rev Med Virol. 2025. PMID: 40346732 Free PMC article. Review.
-
Molecular genetic analysis of orf virus: a poxvirus that has adapted to skin.Viruses. 2015 Mar 23;7(3):1505-39. doi: 10.3390/v7031505. Viruses. 2015. PMID: 25807056 Free PMC article. Review.
References
-
- Abdussalam, M., and V. E. Cosslett. 1957. Contagious pustular dermatitis virus I. Studies on morphology. J. Comp. Path. 67. - PubMed
-
- Adrian, M., J. Dubochet, S. D. Fuller, and J. R. Harris. 1998. Cryo-negative staining. Micron 29:145-160. - PubMed
-
- Adrian, M., J. Dubochet, J. Lepault, and A. W. McDowall. 1984. Cryo-electron microscopy of viruses. Nature 308:32-36. - PubMed
-
- Buttner, M., and H. J. Rziha. 2002. Parapoxviruses: from the lesion to the viral genome. J. Vet. Med. B 49:7-16. - PubMed
-
- Czerny, C. P., S. Johann, L. Holzle, and H. Meyer. 1994. Epitope detection in the envelope of intracellular naked orthopox viruses and identification of encoding genes. Virology 200:764-777. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

