Porcine arterivirus infection of alveolar macrophages is mediated by sialic acid on the virus
- PMID: 15254181
- PMCID: PMC446125
- DOI: 10.1128/JVI.78.15.8094-8101.2004
Porcine arterivirus infection of alveolar macrophages is mediated by sialic acid on the virus
Abstract
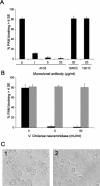
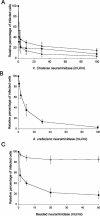
Recently, we showed that porcine sialoadhesin (pSn) mediates internalization of the arterivirus porcine reproductive and respiratory syndrome virus (PRRSV) in alveolar macrophages (Vanderheijden et al., J. Virol. 77:8207-8215, 2003). In rodents and humans, sialoadhesin, or Siglec-1, has been described as a macrophage-restricted molecule and to specifically bind sialic acid moieties. In the current study, we investigated whether pSn is a sialic acid binding protein and, whether so, whether this property is important for its function as a PRRSV receptor. Using untreated and neuraminidase-treated sheep erythrocytes, we showed that pSn binds sialic acid. Furthermore, pSn-specific monoclonal antibody 41D3, which blocks PRRSV infection, inhibited this interaction. PRRSV attachment to and infection of porcine alveolar macrophages (PAM) were both shown to be dependent on the presence of sialic acid on the virus: neuraminidase treatment of virus but not of PAM blocked infection and reduced attachment. Enzymatic removal of all N-linked glycans on the virus with N-glycosidase F reduced PRRSV infection, while exclusive removal of nonsialylated N-linked glycans of the high-mannose type with endoglycosidase H had no significant effect. Free sialyllactose and sialic acid containing (neo)glycoproteins reduced infection, while lactose and (neo)glycoproteins devoid of sialic acids had no significant effect. Studies with linkage-specific neuraminidases and lectins indicated that alpha2-3- and alpha2-6-linked sialic acids on the virion are important for PRRSV infection of PAM. From these results, we conclude that pSn is a sialic acid binding lectin and that interactions between sialic acid on the PRRS virion and pSn are essential for PRRSV infection of PAM.
Figures

Similar articles
-
Porcine arterivirus attachment to the macrophage-specific receptor sialoadhesin is dependent on the sialic acid-binding activity of the N-terminal immunoglobulin domain of sialoadhesin.J Virol. 2007 Sep;81(17):9546-50. doi: 10.1128/JVI.00569-07. Epub 2007 Jun 13. J Virol. 2007. PMID: 17567703 Free PMC article.
-
Porcine reproductive and respiratory syndrome virus attachment is mediated by the N-terminal domain of the sialoadhesin receptor.Vet Microbiol. 2010 Jul 14;143(2-4):371-8. doi: 10.1016/j.vetmic.2009.11.006. Epub 2009 Dec 6. Vet Microbiol. 2010. PMID: 19969429
-
Additive inhibition of porcine reproductive and respiratory syndrome virus infection with the soluble sialoadhesin and CD163 receptors.Virus Res. 2014 Jan 22;179:85-92. doi: 10.1016/j.virusres.2013.11.008. Epub 2013 Nov 15. Virus Res. 2014. PMID: 24246307
-
PRRS virus receptors and their role for pathogenesis.Vet Microbiol. 2015 Jun 12;177(3-4):229-41. doi: 10.1016/j.vetmic.2015.04.002. Epub 2015 Apr 13. Vet Microbiol. 2015. PMID: 25912022 Review.
-
Porcine reproductive and respiratory syndrome virus entry into the porcine macrophage.J Gen Virol. 2010 Jul;91(Pt 7):1659-67. doi: 10.1099/vir.0.020503-0. Epub 2010 Apr 21. J Gen Virol. 2010. PMID: 20410315 Review.
Cited by
-
Recent Advances in PRRS Virus Receptors and the Targeting of Receptor-Ligand for Control.Vaccines (Basel). 2021 Apr 7;9(4):354. doi: 10.3390/vaccines9040354. Vaccines (Basel). 2021. PMID: 33916997 Free PMC article. Review.
-
Porcine sialoadhesin: a newly identified xenogeneic innate immune receptor.Am J Transplant. 2012 Dec;12(12):3272-82. doi: 10.1111/j.1600-6143.2012.04247.x. Epub 2012 Sep 7. Am J Transplant. 2012. PMID: 22958948 Free PMC article.
-
Transcriptome sequencing of microglial cells stimulated with TLR3 and TLR4 ligands.BMC Genomics. 2015 Jul 10;16(1):517. doi: 10.1186/s12864-015-1728-5. BMC Genomics. 2015. PMID: 26159724 Free PMC article.
-
Porcine arterivirus attachment to the macrophage-specific receptor sialoadhesin is dependent on the sialic acid-binding activity of the N-terminal immunoglobulin domain of sialoadhesin.J Virol. 2007 Sep;81(17):9546-50. doi: 10.1128/JVI.00569-07. Epub 2007 Jun 13. J Virol. 2007. PMID: 17567703 Free PMC article.
-
Membrane proteins of arterivirus particles: structure, topology, processing and function.Virus Res. 2014 Dec 19;194:16-36. doi: 10.1016/j.virusres.2014.09.010. Epub 2014 Sep 30. Virus Res. 2014. PMID: 25278143 Free PMC article. Review.
References
-
- Barnes, Y. C., T. P. Skelton, I. Stamenkovic, and D. C. Sgroi. 1999. Sialylation of the sialic acid binding lectin sialoadhesin regulates its ability to mediate cell adhesion. Blood 93:1245-1252. - PubMed
-
- Barretto, N., L. K. Hallak, and M. E. Peeples. 2003. Neuraminidase treatment of respiratory syncytial virus-infected cells or virions, but not target cells, enhances cell-cell fusion and infection. Virology 313:33-42. - PubMed
-
- Benfield, D. A., E. Nelson, J. E. Collins, L. Harris, S. M. Goyal, D. Robinson, W. T. Christianson, R. B. Morrison, D. Gorcyca, and D. Chladek. 1992. Characterisation of swine infertility and respiratory syndrome (SIRS) virus (isolate ATCC VR-2332). J. Vet. Diagn. Investig. 4:127-133. - PubMed
-
- Cavanagh, D. 1997. Nidovirales: a new order comprising Coronaviridae and Arteriviridae. Arch. Virol. 142:629-633. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

