Role of nucleolin in human parainfluenza virus type 3 infection of human lung epithelial cells
- PMID: 15254186
- PMCID: PMC446124
- DOI: 10.1128/JVI.78.15.8146-8158.2004
Role of nucleolin in human parainfluenza virus type 3 infection of human lung epithelial cells
Abstract
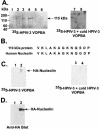
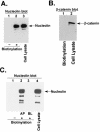
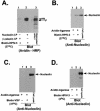
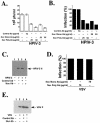
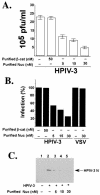
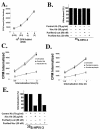
Human parainfluenza virus type 3 (HPIV-3) is an airborne pathogen that infects human lung epithelial cells from the apical (luminal) plasma membrane domain. In the present study, we have identified cell surface-expressed nucleolin as a cellular cofactor required for the efficient cellular entry of HPIV-3 into human lung epithelial A549 cells. Nucleolin was enriched on the apical cell surface domain of A549 cells, and HPIV-3 interacted with nucleolin during entry. The importance of nucleolin during HPIV-3 replication was borne out by the observation that HPIV-3 replication was significantly inhibited following (i). pretreatment of cells with antinucleolin antibodies and (ii). preincubation of HPIV-3 with purified nucleolin prior to its addition to the cells. Moreover, HPIV-3 cellular internalization and attachment assays performed in the presence of antinucleolin antibodies and purified nucleolin revealed the requirement of nucleolin during HPIV-3 internalization but not during attachment. Thus, these results suggest that nucleolin expressed on the surfaces of human lung epithelial A549 cells plays an important role during HPIV-3 cellular entry.
Figures

References
-
- Ah-Type, C., K. Schwartz, E. Huberman, E. Carlin, and A. Moscona. 1999. Virus-receptor interactions of human parainfluenza viruses type 1, 2, and 3. Microb. Pathog. 27:329-336. - PubMed
-
- Barton, E., C. Forrest, J. Connolly, J. Chappel, Y. Liu, F. Schnell, A. Nusrat, C. Parkos, and T. Dermody. 2001. Junction adhesion molecule is a receptor for reovirus. Cell 104:441-451. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

