Hepatitis C virus targets DC-SIGN and L-SIGN to escape lysosomal degradation
- PMID: 15254204
- PMCID: PMC446128
- DOI: 10.1128/JVI.78.15.8322-8332.2004
Hepatitis C virus targets DC-SIGN and L-SIGN to escape lysosomal degradation
Abstract
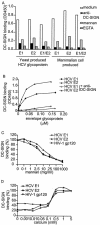
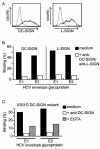
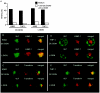
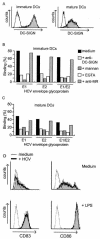
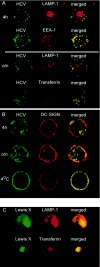
Hepatitis C virus (HCV) is a major health problem. However, the mechanism of hepatocyte infection is largely unknown. We demonstrate that the dendritic cell (DC)-specific C-type lectin DC-SIGN and its liver-expressed homologue L-SIGN/DC-SIGNR are important receptors for HCV envelope glycoproteins E1 and E2. Mutagenesis analyses demonstrated that both HCV E1 and E2 bind the same binding site on DC-SIGN as the pathogens human immunodeficiency virus type 1 (HIV-1) and mycobacteria, which is distinct from the cellular ligand ICAM-3. HCV virus-like particles are efficiently captured and internalized by DCs through binding of DC-SIGN. Antibodies against DC-SIGN specifically block HCV capture by both immature and mature DCs, demonstrating that DC-SIGN is the major receptor on DCs. Interestingly, internalized HCV virus-like particles were targeted to nonlysosomal compartments within immature DCs, where they are protected from lysosomal degradation in a manner similar to that demonstrated for HIV-1. Lewis X antigen, another ligand of DC-SIGN, was internalized to lysosomes, demonstrating that the internalization pathway of DC-SIGN-captured ligands may depend on the structure of the ligand. Our results suggest that HCV may target DC-SIGN to "hide" within DCs and facilitate viral dissemination. L-SIGN, expressed by THP-1 cells, internalized HCV particles into similar nonlysosomal compartments, suggesting that L-SIGN on liver sinusoidal endothelial cells may capture HCV from blood and transmit it to hepatocytes, the primary target for HCV. We therefore conclude that both DCs and liver sinusoidal endothelial cells may act as reservoirs for HCV and that the C-type lectins DC-SIGN and L-SIGN, as important HCV receptors, may represent a molecular target for clinical intervention in HCV infection.
Figures

Similar articles
-
DC-SIGN: binding receptors for hepatitis C virus.Chin Med J (Engl). 2004 Sep;117(9):1395-400. Chin Med J (Engl). 2004. PMID: 15377434 Review.
-
C-type lectins L-SIGN and DC-SIGN capture and transmit infectious hepatitis C virus pseudotype particles.J Biol Chem. 2004 Jul 30;279(31):32035-45. doi: 10.1074/jbc.M402296200. Epub 2004 May 27. J Biol Chem. 2004. PMID: 15166245
-
L-SIGN (CD209L) and DC-SIGN (CD209) mediate transinfection of liver cells by hepatitis C virus.Proc Natl Acad Sci U S A. 2004 Sep 28;101(39):14067-72. doi: 10.1073/pnas.0405695101. Epub 2004 Sep 15. Proc Natl Acad Sci U S A. 2004. PMID: 15371595 Free PMC article.
-
Infection of dendritic cells (DCs), not DC-SIGN-mediated internalization of human immunodeficiency virus, is required for long-term transfer of virus to T cells.J Virol. 2006 Mar;80(6):2949-57. doi: 10.1128/JVI.80.6.2949-2957.2006. J Virol. 2006. PMID: 16501104 Free PMC article.
-
DC-SIGN (dendritic cell-specific ICAM-grabbing non-integrin) and DC-SIGN-related (DC-SIGNR): friend or foe?Clin Sci (Lond). 2003 Apr;104(4):437-46. Clin Sci (Lond). 2003. PMID: 12653690 Review.
Cited by
-
Human lectins and their roles in viral infections.Molecules. 2015 Jan 29;20(2):2229-71. doi: 10.3390/molecules20022229. Molecules. 2015. PMID: 25642836 Free PMC article. Review.
-
Human cytomegalovirus entry into dendritic cells occurs via a macropinocytosis-like pathway in a pH-independent and cholesterol-dependent manner.PLoS One. 2012;7(4):e34795. doi: 10.1371/journal.pone.0034795. Epub 2012 Apr 9. PLoS One. 2012. PMID: 22496863 Free PMC article.
-
DC-SIGN mediated sphingomyelinase-activation and ceramide generation is essential for enhancement of viral uptake in dendritic cells.PLoS Pathog. 2011 Feb;7(2):e1001290. doi: 10.1371/journal.ppat.1001290. Epub 2011 Feb 17. PLoS Pathog. 2011. PMID: 21379338 Free PMC article.
-
The allele 4 of neck region liver-lymph node-specific ICAM-3-grabbing integrin variant is associated with spontaneous clearance of hepatitis C virus and decrease of viral loads.Clin Microbiol Infect. 2014 May;20(5):O325-32. doi: 10.1111/1469-0691.12403. Epub 2013 Nov 6. Clin Microbiol Infect. 2014. PMID: 24283933 Free PMC article.
-
Impact of polymorphisms in the DC-SIGNR neck domain on the interaction with pathogens.Virology. 2006 Apr 10;347(2):354-63. doi: 10.1016/j.virol.2005.11.033. Epub 2006 Jan 18. Virology. 2006. PMID: 16413044 Free PMC article.
References
-
- Alter, H. J., and L. B. Seeff. 2000. Recovery, persistence, and sequelae in hepatitis C virus infection: a perspective on long-term outcome. Semin. Liver Dis. 20:17-35. - PubMed
-
- Auffermann-Gretzinger, S., E. B. Keeffe, and S. Levy. 2001. Impaired dendritic cell maturation in patients with chronic, but not resolved, hepatitis C virus infection. Blood 97:3171-3176. - PubMed
-
- Bain, C., A. Fatmi, F. Zoulim, J. P. Zarski, C. Trepo, and G. Inchauspe. 2001. Impaired allostimulatory function of dendritic cells in chronic hepatitis C infection. Gastroenterology 120:512-524. - PubMed
-
- Bashirova, A. A., T. B. Geijtenbeek, G. C. van Duijnhoven, S. J. van Vliet, J. B. Eilering, M. P. Martin, L. Wu, T. D. Martin, N. Viebig, P. A. Knolle, V. N. KewalRamani, Y. Van Kooyk, and M. Carrington. 2001. A dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin (DC-SIGN)-related protein is highly expressed on human liver sinusoidal endothelial cells and promotes HIV-1 infection. J. Exp. Med. 193:671-678. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

