mTOR is essential for growth and proliferation in early mouse embryos and embryonic stem cells
- PMID: 15254238
- PMCID: PMC444840
- DOI: 10.1128/MCB.24.15.6710-6718.2004
mTOR is essential for growth and proliferation in early mouse embryos and embryonic stem cells
Abstract
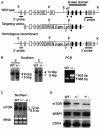
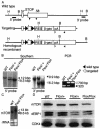
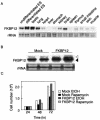
TOR is a serine-threonine kinase that was originally identified as a target of rapamycin in Saccharomyces cerevisiae and then found to be highly conserved among eukaryotes. In Drosophila melanogaster, inactivation of TOR or its substrate, S6 kinase, results in reduced cell size and embryonic lethality, indicating a critical role for the TOR pathway in cell growth control. However, the in vivo functions of mammalian TOR (mTOR) remain unclear. In this study, we disrupted the kinase domain of mouse mTOR by homologous recombination. While heterozygous mutant mice were normal and fertile, homozygous mutant embryos died shortly after implantation due to impaired cell proliferation in both embryonic and extraembryonic compartments. Homozygous blastocysts looked normal, but their inner cell mass and trophoblast failed to proliferate in vitro. Deletion of the C-terminal six amino acids of mTOR, which are essential for kinase activity, resulted in reduced cell size and proliferation arrest in embryonic stem cells. These data show that mTOR controls both cell size and proliferation in early mouse embryos and embryonic stem cells.
Figures




References
-
- Brown, E. J., M. W. Albers, T. B. Shin, K. Ichikawa, C. T. Keith, W. S. Lane, and S. L. Schreiber. 1994. A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature 369:756-758. - PubMed
-
- Brown, E. J., P. A. Beal, C. T. Keith, J. Chen, T. B. Shin, and S. L. Schreiber. 1995. Control of p70 s6 kinase by kinase activity of FRAP in vivo. Nature 377:441-446. - PubMed
-
- Brunn, G. J., C. C. Hudson, A. Sekulic, J. M. Williams, H. Hosoi, P. J. Houghton, J. C. Lawrence, Jr., and R. T. Abraham. 1997. Phosphorylation of the translational repressor PHAS-I by the mammalian target of rapamycin. Science 277:99-101. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
