NrCAM coupling to the cytoskeleton depends on multiple protein domains and partitioning into lipid rafts
- PMID: 15254265
- PMCID: PMC519160
- DOI: 10.1091/mbc.e04-03-0171
NrCAM coupling to the cytoskeleton depends on multiple protein domains and partitioning into lipid rafts
Abstract
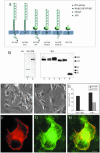
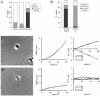
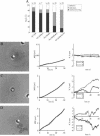
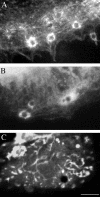
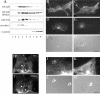
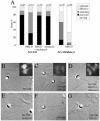
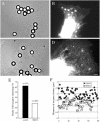
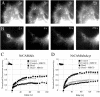
NrCAM is a cell adhesion molecule of the L1 family that is implicated in the control of axonal growth. Adhesive contacts may promote advance of the growth cone by triggering the coupling of membrane receptors with the F-actin retrograde flow. We sought to understand the mechanisms leading to clutching the F-actin at the site of ligand-mediated clustering of NrCAM. Using optical tweezers and single particle tracking of beads coated with the ligand TAG-1, we analyzed the mobility of NrCAM-deletion mutants transfected in a neuroblastoma cell line. Deletion of the cytoplasmic tail did not prevent the coupling of NrCAM to the actin flow. An additional deletion of the FNIII domains to remove cis-interactions, was necessary to abolish the rearward movement of TAG-1 beads, which instead switched to a stationary behavior. Next, we showed that the actin-dependent retrograde movement of NrCAM required partitioning into lipid rafts as indicated by cholesterol depletion experiments using methyl-beta-cyclodextrin. Recruitment of the raft component caveolin-1 was induced at the adhesive contact between the cell surface and TAG-1 beads, indicating that enlarged rafts were generated. Photobleaching experiments showed that the lateral mobility of NrCAM increased with raft dispersion in these contact areas, further suggesting that TAG-1-coated beads induced the coalescence of lipid rafts. In conclusion, we propose that anchoring of NrCAM with the retrograde actin flow can be triggered by adhesive contacts via cooperative processes including interactions with the cytoplasmic tail, formation of cis-complex via the FNIII repeats, and lipid raft aggregation.
Figures

Similar articles
-
NrCAM, cerebellar granule cell receptor for the neuronal adhesion molecule F3, displays an actin-dependent mobility in growth cones.J Cell Sci. 1999 Sep;112 Pt 18:3015-27. doi: 10.1242/jcs.112.18.3015. J Cell Sci. 1999. PMID: 10462518
-
F-actin dynamics control segregation of the TCR signaling cascade to clustered lipid rafts.Eur J Immunol. 2002 Feb;32(2):435-46. doi: 10.1002/1521-4141(200202)32:2<435::AID-IMMU435>3.0.CO;2-H. Eur J Immunol. 2002. PMID: 11813162
-
Agrin triggers the clustering of raft-associated acetylcholine receptors through actin cytoskeleton reorganization.Biol Cell. 2011 Jun;103(6):287-301. doi: 10.1042/BC20110018. Biol Cell. 2011. PMID: 21524273
-
Spatial control of actin-based motility through plasmalemmal PtdIns(4,5)P2-rich raft assemblies.Biochem Soc Symp. 2005;(72):119-27. doi: 10.1042/bss0720119. Biochem Soc Symp. 2005. PMID: 15649136 Review.
-
Cytoskeleton-membrane interactions in membrane raft structure.Cell Mol Life Sci. 2009 Jul;66(14):2319-28. doi: 10.1007/s00018-009-0022-6. Epub 2009 Apr 16. Cell Mol Life Sci. 2009. PMID: 19370312 Free PMC article. Review.
Cited by
-
Characterisation of detergent-insoluble membranes in pollen tubes of Nicotiana tabacum (L.).Biol Open. 2015 Feb 20;4(3):378-99. doi: 10.1242/bio.201410249. Biol Open. 2015. PMID: 25701665 Free PMC article.
-
NrCAM is a marker for substrate-selective activation of ADAM10 in Alzheimer's disease.EMBO Mol Med. 2019 Apr;11(4):e9695. doi: 10.15252/emmm.201809695. EMBO Mol Med. 2019. PMID: 30833305 Free PMC article.
-
L1-mediated branching is regulated by two ezrin-radixin-moesin (ERM)-binding sites, the RSLE region and a novel juxtamembrane ERM-binding region.J Neurosci. 2005 Jan 12;25(2):395-403. doi: 10.1523/JNEUROSCI.4097-04.2005. J Neurosci. 2005. PMID: 15647482 Free PMC article.
-
Characterization of lipid rafts from Medicago truncatula root plasma membranes: a proteomic study reveals the presence of a raft-associated redox system.Plant Physiol. 2007 May;144(1):402-18. doi: 10.1104/pp.106.094102. Epub 2007 Mar 2. Plant Physiol. 2007. PMID: 17337521 Free PMC article.
-
Fast turnover of L1 adhesions in neuronal growth cones involving both surface diffusion and exo/endocytosis of L1 molecules.Mol Biol Cell. 2007 Aug;18(8):3131-43. doi: 10.1091/mbc.e06-12-1101. Epub 2007 May 30. Mol Biol Cell. 2007. PMID: 17538021 Free PMC article.
References
-
- Bennett, V., and Chen, L. (2001). Ankyrins and cellular targeting of diverse membrane proteins to physiological sites. Curr. Opin. Cell Biol. 13, 61-67. - PubMed
-
- Brümmendorf, T., and Rathjen, F.G. (1996). Structure/function relationships of axon-associated adhesion receptors of the immunoglobulin superfamily. Curr. Opin. Neurobiol. 6, 584-593. - PubMed
-
- Choquet, D., Felsenfeld, D.P., and Sheetz, M.P. (1997). Extracellular matrix rigidity causes strengthening of integrin-cytoskeleton linkages. Cell 88, 39-48. - PubMed
-
- Coussen, F., Choquet, D., Sheetz, M.P., and Erickson, H.P. (2002). Trimers of the fibronectin cell adhesion domain localize to actin filament bundles and undergo rearward translocation. J. Cell Sci. 115, 2581-2590. - PubMed
-
- Dahlin-Huppe, K., Berglund, E.O., Ranscht, B., and W.B. Stallcup. (1997). Mutational analysis of the L1 neuronal cell adhesion molecule identifies membrane-proximal amino acids of the cytoplasmic domain that are required for cytoskeletal anchorage. Mol. Cell Neurosci. 9, 144-156. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

