Human eosinophil chemotaxis and selective in vivo recruitment by sphingosine 1-phosphate
- PMID: 15254297
- PMCID: PMC503758
- DOI: 10.1073/pnas.0401439101
Human eosinophil chemotaxis and selective in vivo recruitment by sphingosine 1-phosphate
Abstract
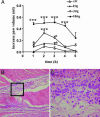
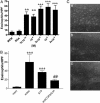
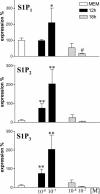
Sphingosine 1-phosphate (S1P) is a sphingolipid mediator that is involved in diverse biological functions. Local administration of S1P causes inflammation coupled to a large eosinophil (EO) recruitment in the rat-paw tissue. The inflammatory response is accompanied by an increase in S1P receptors, namely S1P(1), S1P(2), S1P(3), and by an enhanced expression of CCR3, which is the main chemokine receptor known to be involved in EO function. Human EOs constitutively express S1P(1) and, at a lower extent, S1P(2), S1P(3) receptors. S1P in vitro causes cultured human EO migration and an increase in S1P receptor mRNA copies and strongly up-regulates CCR3 and RANTES (regulated on activation, normal T cell-expressed and secreted) message levels; in particular CCR3 is up-regulated 18,000-fold by S1P. A blocking anti-CCR3 Ab inhibits S1P-induced chemotaxis, implying that S1P acts as specific recruiting signal for EOs not only through its own receptors but also through CCR3. These results show that S1P is involved in EO chemotaxis and contribute to shed light on the complex mechanisms underlying EO recruitment in several diseases such as asthma and some malignancies.
Figures

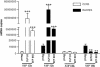
Similar articles
-
In vitro assessment of chemokine receptor-ligand interactions mediating mouse eosinophil migration.J Leukoc Biol. 2002 Jun;71(6):1033-41. J Leukoc Biol. 2002. PMID: 12050190
-
S1P(1) overexpression stimulates S1P-dependent chemotaxis of human CD34+ hematopoietic progenitor cells but strongly inhibits SDF-1/CXCR4-dependent migration and in vivo homing.Mol Immunol. 2008 Nov;46(1):166-71. doi: 10.1016/j.molimm.2008.07.016. Epub 2008 Aug 29. Mol Immunol. 2008. PMID: 18760838
-
Sphingosine 1-phosphate inhibits migration and RANTES production in human bronchial smooth muscle cells.Biochem Biophys Res Commun. 2005 Jun 3;331(2):640-7. doi: 10.1016/j.bbrc.2005.03.223. Biochem Biophys Res Commun. 2005. PMID: 15850807
-
Sphingosine 1-phosphate and its type 1 G protein-coupled receptor: trophic support and functional regulation of T lymphocytes.J Leukoc Biol. 2004 Jul;76(1):30-5. doi: 10.1189/jlb.1103567. Epub 2004 Feb 24. J Leukoc Biol. 2004. PMID: 14982946 Review.
-
Tipping the gatekeeper: S1P regulation of endothelial barrier function.Trends Immunol. 2007 Mar;28(3):102-7. doi: 10.1016/j.it.2007.01.007. Epub 2007 Feb 5. Trends Immunol. 2007. PMID: 17276731 Review.
Cited by
-
Novel uses for anti-platelet agents as anti-inflammatory drugs.Br J Pharmacol. 2007 Dec;152(7):987-1002. doi: 10.1038/sj.bjp.0707364. Epub 2007 Jul 2. Br J Pharmacol. 2007. PMID: 17603547 Free PMC article. Review.
-
FTY720 regulates bone marrow egress of eosinophils and modulates late-phase skin reaction in mice.Am J Pathol. 2010 Oct;177(4):1881-7. doi: 10.2353/ajpath.2010.100119. Epub 2010 Aug 27. Am J Pathol. 2010. PMID: 20802177 Free PMC article.
-
Sphingosine-1-phosphate activates mouse vagal airway afferent C-fibres via S1PR3 receptors.J Physiol. 2019 Apr;597(7):2007-2019. doi: 10.1113/JP277521. Epub 2019 Feb 21. J Physiol. 2019. PMID: 30793318 Free PMC article.
-
HMGB1 Facilitated Macrophage Reprogramming towards a Proinflammatory M1-like Phenotype in Experimental Autoimmune Myocarditis Development.Sci Rep. 2016 Feb 22;6:21884. doi: 10.1038/srep21884. Sci Rep. 2016. Retraction in: Sci Rep. 2022 Apr 8;12(1):5931. doi: 10.1038/s41598-022-10210-2. PMID: 26899795 Free PMC article. Retracted.
-
Role of Sphingosine-1-Phosphate in Mast Cell Functions and Asthma and Its Regulation by Non-Coding RNA.Front Immunol. 2017 May 22;8:587. doi: 10.3389/fimmu.2017.00587. eCollection 2017. Front Immunol. 2017. PMID: 28588581 Free PMC article. Review.
References
-
- Yatomi, Y., Ohmori, T., Rile, G., Kazama, F., Okamoto, H., Sano, T., Satoh, K., Kume, S., Tigyi, G., Igarashi, Y. & Ozaki, Y. (2000) Blood 96, 3431–3438. - PubMed
-
- Chun, J., Goetzl, E. J., Hla, T., Igarashi, Y., Lynch, K. R., Moolenaar, W., Pyne, S. & Tigyi, G. (2002) Pharmacol. Rev. 54, 265–269. - PubMed
-
- Spiegel, S. & Milstien, S. (2003) Nat. Rev. Mol. Cell Biol. 4, 397–407. - PubMed
-
- Spiegel, S., English, D. & Milstien, S. (2002) Trends Cell Biol. 12, 236–242. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

