Activation of cytokines and NF-kappa B in corneal epithelial cells infected by respiratory syncytial virus: potential relevance in ocular inflammation and respiratory infection
- PMID: 15256003
- PMCID: PMC481065
- DOI: 10.1186/1471-2180-4-28
Activation of cytokines and NF-kappa B in corneal epithelial cells infected by respiratory syncytial virus: potential relevance in ocular inflammation and respiratory infection
Abstract
Background: Respiratory syncytial virus (RSV) is a major cause of lower respiratory tract infection, claiming millions of lives annually. The virus infects various cells of the respiratory tract as well as resident inflammatory cells such as macrophages. Infection activates a variety of cellular factors such as cytokines and the pro-inflammatory transcription factor, NF-kappa B, all of which are important players in the respiratory disease. However, the exact natural route of RSV infection and its etiology remain relatively unknown. In this paper, we test the hypothesis that human corneal epithelial cells, which constitute the outermost layer of the cornea, can be infected with RSV, and that the infection leads to the activation of proinflammatory macromolecules.
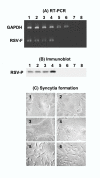
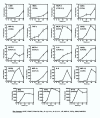
Results: Corneal swabs obtained from pediatric patients with acute respiratory disease were found to contain RSV at a high frequency (43 positive out of 72 samples, i.e., 60%). Primary corneal epithelial cells in tissue culture supported robust infection and productive growth of RSV. Infection resulted in the activation of TNF-alpha, IL-6 and sixteen chemokines as well as NF-kappa B. Three proinflammatory CXC chemokines (MIG, I-TAC, IP-10) underwent the greatest activation.
Conclusions: The ocular epithelium is readily infected by RSV. The pro-inflammatory cytokines are likely to play critical roles in the etiology of inflammation and conjunctivitis commonly seen in pediatric patients with respiratory infections. RSV-eye interactions have important implications in RSV transmission, immunopathology of RSV disease, and in the management of conjunctivitis.
Figures




References
-
- Marriott AC, Easton AJ. Reverse genetics of the Paramyxoviridae. Adv Virus Res. 1999;53:321–340. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

