Aberrant methylation of the maspin promoter is an early event in human breast cancer
- PMID: 15256060
- PMCID: PMC1502109
- DOI: 10.1593/neo.04115
Aberrant methylation of the maspin promoter is an early event in human breast cancer
Abstract
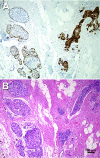
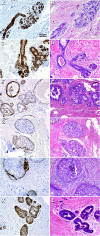
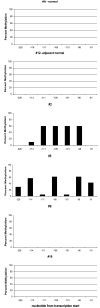
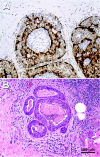
The maspin gene functions as a tumor suppressor in human breasts, and its expression is frequently lost during breast cancer progression. In vitro models of human breast cancer indicate that the loss of maspin expression is closely linked to aberrant methylation of the maspin promoter. We conducted a study on 30 archival ductal carcinoma in situ (DCIS) specimens to determine if aberrant methylation of the maspin promoter occurred in vivo, and whether it occurred early in breast cancer evolution. Healthy tissue obtained from reduction mammoplasty was used as normal control. Results from immunohistochemical analysis indicate that maspin expression is lost in a substantial fraction of DCIS specimens (57%). Bisulfite sequencing of DNA isolated from laser capture-microdissected normal and neoplastic ducts showed that loss of maspin expression was often, but not always, linked to aberrant methylation of the maspin promoter, suggesting that other mechanisms, in addition to aberrant methylation, participate and/or cooperate to silence maspin gene expression. Taken together, these results indicate that aberrant methylation of the maspin promoter is an early event in human breast cancer.
Copyright 2004 Neoplasia Press, Inc.
Figures


References
-
- Baylin SB, Herman JG. DNA hypermethylation in tumorigenesis: epigenetics joins genetics. Trends Genet. 2000;16:168–174. - PubMed
-
- Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet. 2002;3:415–428. - PubMed
-
- Feinberg AP, Cui H, Ohlsson R. DNA methylation and genomic imprinting: insights from cancer into epigenetic mechanisms. Semin Cancer Biol. 2002;12:389–398. - PubMed
-
- Herman JG. Hypermethylation of tumor suppressor genes in cancer. Semin Cancer Biol. 1999;9:359–367. - PubMed
-
- Costello JF, Fruhwald MC, Smiraglia DJ, Rush LJ, Robertson GP, Gao X, Wright FA, Feramisco JD, Peltomaki P, Lang JC, Schuller DE, Yu L, Bloomfield CD, Caligiuri MA, Yates A, Nishikawa R, SuHuang H, Petrelli NJ, Zhang X, O'Dorisio MS, Held WA, Cavenee WK, Plass C. Aberrant CpG-island methylation has non-random and tumour-type-specific patterns [see comments] Nat Genet. 2000;24:132–138. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
