Tal, a Tsg101-specific E3 ubiquitin ligase, regulates receptor endocytosis and retrovirus budding
- PMID: 15256501
- PMCID: PMC478194
- DOI: 10.1101/gad.294904
Tal, a Tsg101-specific E3 ubiquitin ligase, regulates receptor endocytosis and retrovirus budding
Abstract
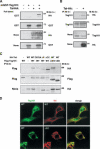
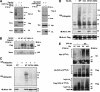
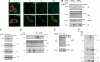
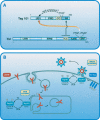
The tumor suppressor gene 101 (tsg101) regulates vesicular trafficking processes in yeast and mammals. We report a novel protein, Tal (Tsg101-associated ligase), whose RING finger is necessary for multiple monoubiquitylation of Tsg101. Bivalent binding of Tsg101 to a tandem tetrapeptide motif (PTAP) and to a central region of Tal is essential for Tal-mediated ubiquitylation of Tsg101. By studying endocytosis of the epidermal growth factor receptor and egress of the human immunodeficiency virus, we conclude that Tal regulates a Tsg101-associated complex responsible for the sorting of cargo into cytoplasm-containing vesicles that bud at the multivesicular body and at the plasma membrane.
Copyright 2004 Cold Spring Harbor Laboratory Press ISSN
Figures




References
-
- Babst M., Odorizzi, G., Estepa, E.J., and Emr, S.D. 2000. Mammalian tumor susceptibility gene 101 (TSG101) and the yeast homologue, Vps23p, both function in late endosomal trafficking. Traffic 1: 248-258. - PubMed
-
- Bache K.G., Raiborg, C., Mehlum, A., and Stenmark, H. 2003b. STAM and Hrs are subunits of a multivalent ubiquitin-binding complex on early endosomes. J. Biol. Chem. 278: 12513-12521. - PubMed
-
- Bishop N. and Woodman, P. 2001. TSG101/mammalian VPS23 and mammalian VPS28 interact directly and are recruited to VPS4-induced endosomes. J. Biol. Chem. 276: 11735-11742. - PubMed
-
- Cadavid A.L., Ginzel, A., and Fischer, J.A. 2000. The function of the Drosophila fat facets deubiquitinating enzyme in limiting photoreceptor cell number is intimately associated with endocytosis. Development 127: 1727-1736. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
