Topological domain structure of the Escherichia coli chromosome
- PMID: 15256503
- PMCID: PMC478196
- DOI: 10.1101/gad.1207504
Topological domain structure of the Escherichia coli chromosome
Abstract
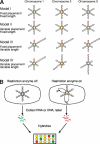
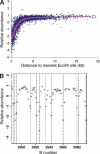
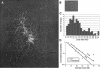
The circular chromosome of Escherichia coli is organized into independently supercoiled loops, or topological domains. We investigated the organization and size of these domains in vivo and in vitro. Using the expression of >300 supercoiling-sensitive genes to gauge local chromosomal supercoiling, we quantitatively measured the spread of relaxation from double-strand breaks generated in vivo and thereby calculated the distance to the nearest domain boundary. In a complementary approach, we gently isolated chromosomes and examined the lengths of individual supercoiled loops by electron microscopy. The results from these two very different methods agree remarkably well. By comparing our results to Monte Carlo simulations of domain organization models, we conclude that domain barriers are not placed stably at fixed sites on the chromosome but instead are effectively randomly distributed. We find that domains are much smaller than previously reported, approximately 10 kb on average. We discuss the implications of these findings and present models for how domain barriers may be generated and displaced during the cell cycle in a stochastic fashion.
Copyright 2004 Cold Spring Harbor Laboratory Press ISSN
Figures




References
-
- Aussel L., Barre, F.X., Aroyo, M., Stasiak, A., Stasiak, A.Z., and Sherratt, D. 2002. FtsK Is a DNA motor protein that activates chromosome dimer resolution by switching the catalytic state of the XerC and XerD recombinases. Cell 108: 195-205. - PubMed
-
- Blattner F.R., Plunkett III, G., Bloch, C.A., Perna, N.T., Burland, V., Riley, M., Collado-Vides, J., Glasner, J.D., Rode, C.K., Mayhew, G.F., et al. 1997. The complete genome sequence of Escherichia coli K-12. Science 277: 1453-1474. - PubMed
-
- Bliska J.B. and Cozzarelli, N.R. 1987. Use of site-specific recombination as a probe of DNA structure and metabolism in vivo. J. Mol. Biol. 194: 205-218. - PubMed
-
- Case R., Chang, Y., Smith, S., Gore, J., Cozzarelli, N., and Bustamante, C. 2004. The bacterial condensin MukBEF compacts DNA into a repetitive, stable structure. Science (in press). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
