Comparative evolutionary genomics of androgen-binding protein genes
- PMID: 15256509
- PMCID: PMC509260
- DOI: 10.1101/gr.2540304
Comparative evolutionary genomics of androgen-binding protein genes
Abstract
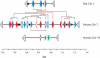
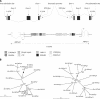
Allelic variation within the mouse androgen-binding protein (ABP) alpha subunit gene (Abpa) has been suggested to promote assortative mating and thus prezygotic isolation. This is consistent with the elevated evolutionary rates observed for the Abpa gene, and the Abpb and Abpg genes whose products (ABPbeta and ABPgamma) form heterodimers with ABPalpha. We have investigated the mouse sequence that contains the three Abpa/b/g genes, and orthologous regions in rat, human, and chimpanzee genomes. Our studies reveal extensive "remodeling" of this region: Duplication rates of Abpa-like and Abpbg-like genes in mouse are >2 orders of magnitude higher than the average rate for all mouse genes; synonymous nucleotide substitution rates are twofold higher; and the Abpabg genomic region has expanded nearly threefold since divergence of the rodents. During this time, one in six amino acid sites in ABPbetagamma-like proteins appear to have been subject to positive selection; these may constitute a site of interaction with receptors or ligands. Greater adaptive variation among Abpbg-like sequences than among Abpa-like sequences suggests that assortative mating preferences are more influenced by variation in Abpbg-like genes. We propose a role for ABPalpha/beta/gamma proteins as pheromones, or in modulating odorant detection. This would account for the extraordinary adaptive evolution of these genes, and surrounding genomic regions, in murid rodents.
Copyright 2004 Cold Spring Harbor Laboratory Press ISSN
Figures



References
-
- Adkins, R.M., Gelke, E.L., Rowe, D., and Honeycutt, R.L. 2001. Molecular phylogeny and divergence time estimates for major rodent groups: Evidence from multiple genes. Mol. Biol. Evol. 18: 777–791. - PubMed
-
- Bains, W. 1992. Local sequence dependence of rate of base replacement in mammals. Mutat. Res. 267: 43–54. - PubMed
-
- Beato, M. and Baier, R. 1975. Binding of progesterone to the proteins of the uterine luminal fluid. Identification of uteroglobin as the binding protein. Biochim. Biophys. Acta 392: 346–354. - PubMed
-
- Bimova, B., Karn, R.C., and Pialek, J. 2004. The role of salivary androgen-binding protein in reproductive isolation between two subspecies of house mouse: Mus musculus musculus and Mus musculus domesticus. Biol. J. Linn. Soc. (in press).
WEB SITE REFERENCES
-
- http://ftp.genome.washington.edu/cgi-bin/RepeatMasker; RepeatMasker.
-
- http://genome.cse.ucsc.edu/; UCSC genome browser.
-
- http://www.ensembl.org/; Ensembl genome browser.
-
- http://www.expasy.org/spdbv/; Swiss-PDBviewer.
-
- http://www.povray.org/; POVRAY, graphical representation programs.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
