Mitogen-activated protein kinase pathways defend against bacterial pore-forming toxins
- PMID: 15256590
- PMCID: PMC503732
- DOI: 10.1073/pnas.0404073101
Mitogen-activated protein kinase pathways defend against bacterial pore-forming toxins
Abstract
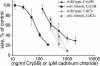
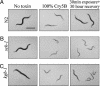
Cytolytic pore-forming toxins are important for the virulence of many disease-causing bacteria. How target cells molecularly respond to these toxins and whether or not they can mount a defense are poorly understood. By using microarrays, we demonstrate that the nematode Caenorhabditis elegans responds robustly to Cry5B, a member of the pore-forming Crystal toxin family made by Bacillus thuringiensis. This genomic response is distinct from that seen with a different stressor, the heavy metal cadmium. A p38 mitogen-activated protein kinase (MAPK) kinase and a c-Jun N-terminal-like MAPK are both transcriptionally up-regulated by Cry5B. Moreover, both MAPK pathways are functionally important because elimination of either leads to animals that are (i) hypersensitive to a low, chronic dose of toxin and (ii) hypersensitive to a high, brief dose of toxin such that the animal might naturally encounter in the wild. These results extend to mammalian cells because inhibition of p38 results in the hypersensitivity of baby hamster kidney cells to aerolysin, a pore-forming toxin that targets humans. Furthermore, we identify two downstream transcriptional targets of the p38 MAPK pathway, ttm-1 and ttm-2, that are required for defense against Cry5B. Our data demonstrate that cells defend against pore-forming toxins by means of conserved MAPK pathways.
Figures




Comment in
-
MAPping innate immunity.Proc Natl Acad Sci U S A. 2004 Aug 31;101(35):12781-2. doi: 10.1073/pnas.0404890101. Epub 2004 Aug 24. Proc Natl Acad Sci U S A. 2004. PMID: 15328410 Free PMC article. No abstract available.
References
-
- van der Goot, F. G. (2003) in Bacterial Protein Toxins, eds. Burns, D. L., Barbieri, J. T., Iglewski, B. H. & Rappuoli, R. (Am. Soc. Microbiol., Washington, DC), pp. 189–202.
-
- Alouf, J. E. (2003) Folia Microbiol. (Praha) 48, 5–16. - PubMed
-
- Alouf, J. E. (2001) Curr. Top. Microbiol. Immunol. 257, 1–14. - PubMed
-
- Huffman, D. L., Bischof, L. J., Griffitts, J. S. & Aroian, R. V. (2004) Int. J. Med. Microbiol. 293, 599–607. - PubMed
-
- de Maagd, R. A., Bravo, A., Berry, C., Crickmore, N. & Schnepf, H. E. (2003) Annu. Rev. Genet. 37, 409–433. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

