Innervation of ectopic endometrium in a rat model of endometriosis
- PMID: 15256593
- PMCID: PMC491992
- DOI: 10.1073/pnas.0403663101
Innervation of ectopic endometrium in a rat model of endometriosis
Abstract
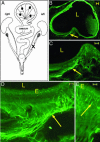
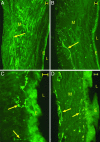
Endometriosis (ENDO) is a disorder in which vascularized growths of endometrial tissue occur outside the uterus. Its symptoms include reduced fertility and severe pelvic pain. Mechanisms that maintain the ectopic growths and evoke symptoms are poorly understood. One factor not yet considered is that the ectopic growths develop their own innervation. Here, we tested the hypothesis that the growths develop both an autonomic and a sensory innervation. We used a rat model of surgically induced ENDO whose growths mimic those in women. Furthermore, similar to women with ENDO, such rats exhibit reduced fertility and increased pelvic nociception. The ENDO was induced by autotransplanting, on mesenteric cascade arteries, small pieces of uterus that formed vascularized cysts. The cysts and healthy uterus were harvested from proestrous rats and immunostained using the pan-neuronal marker PGP9.5 and specific markers for calcitonin gene-related peptide (CGRP) (sensory C and A delta fibers), substance P (SP) (sensory C and A delta fibers) and vesicular monoamine transporter (sympathetic fibers). Cysts (like the uterus) were robustly innervated, with many PGP9.5-stained neurites accompanying blood vessels and extending into nearby luminal epithelial layers. CGRP-, SP-, and vesicular monoamine transporter-immunostained neurites also were observed, with CGRP and SP neurites extending the furthest into the cyst lining. These results demonstrate that ectopic endometrial growths develop an autonomic and sensory innervation. This innervation could contribute not only to symptoms associated with ENDO but also to maintenance of the ectopic growths.
Figures

References
-
- Yoshinaga, K. & Parrott, E. C., eds. (2002) Ann. N.Y. Acad. Sci. 955, 1–408. - PubMed
-
- Parazzini, F., Cipriani, S., Moroni, S. & Crosignani, P. G. (2001) Hum. Reprod. 16, 2668–2671. - PubMed
-
- Chapron, C., Fauconnier, A., Dubuisson, J. B., Barakat, H., Vieira, M. & Breart, G. (2003) Hum. Reprod. 18, 760–766. - PubMed
-
- Wu, M. Y. & Ho, H. N. (2003) Am. J. Reprod. Immunol. 49, 285–296. - PubMed
-
- Ferretti, A., Boschi, E., Stefani, A., Spiga, S., Romanelli, M., Lemmi, M., Giovannetti, A., Longoni, B. & Mosca, F. (2003) Life Sci. 73, 1985–1994. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

