A point mutation in the regulatory light chain reduces the step size of skeletal muscle myosin
- PMID: 15256600
- PMCID: PMC503728
- DOI: 10.1073/pnas.0401699101
A point mutation in the regulatory light chain reduces the step size of skeletal muscle myosin
Abstract
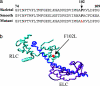
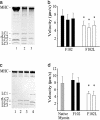
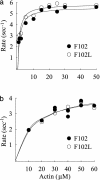
Current evidence favors the theory that, when the globular motor domain of myosin attaches to actin, the light chain binding domain or "lever arm" rotates, and thereby generates movement of actin filaments. Myosin is uniquely designed for such a role in that a long alpha-helix (approximately 9 nm) extending from the C terminus of the catalytic core is stabilized by two calmodulin-like molecules, the regulatory light chain (RLC) and the essential light chain (ELC). Here, we introduce a single-point mutation into the skeletal myosin RLC, which results in a large (approximately 50%) reduction in actin filament velocity (V(actin)) without any loss in actin-activated MgATPase activity. Single-molecule analysis of myosin by optical trapping showed a comparable 2-fold reduction in unitary displacement or step size (d), without a significant change in the duration of the strongly attached state (tau(on)) after the power stroke. Assuming that V(actin) approximately d/tau(on), we can account for the change in velocity primarily by a change in the step size of the lever arm without incurring any change in the kinetic properties of the mutant myosin. These results suggest that a principal role for the many light chain isoforms in the myosin II class may be to modulate the flexural rigidity of the light chain binding domain to maximize tension development and movement during muscle contraction.
Figures
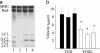
References
-
- Rayment, I., Rypniewski, W. R., Schmidt-Base, K., Smith, R., Tomchick, D. R., Benning, M. M., Winkelmann, D. A., Wesenberg, G. & Holden, H. M. (1993) Science 261, 50–58. - PubMed
-
- Fisher, A. J., Smith, C. A., Thoden, J. B. Smith, R., Sutoh, K., Holden, H. M. & Rayment, I. (1995) Biochemistry 34, 8960–8972. - PubMed
-
- Dominguez, R., Freyzon, Y., Trybus, K. M. & Cohen, C. (1998) Cell 94, 559–571. - PubMed
-
- Rayment, I., Holden, H. M., Whittaker, M., Yohn, C. B., Lorenz, M., Holmes, K. C. & Milligan, R. A. (1993) Science 261, 58–65. - PubMed
-
- Whittaker, M., Wilson-Kubalek, E. M., Smith, J. E., Faust, L., Milligan, R. A. & Sweeney, H. L. (1995) Nature 378, 748–751. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

