Collagenase unwinds triple-helical collagen prior to peptide bond hydrolysis
- PMID: 15257288
- PMCID: PMC514933
- DOI: 10.1038/sj.emboj.7600318
Collagenase unwinds triple-helical collagen prior to peptide bond hydrolysis
Abstract
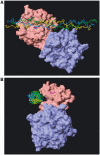
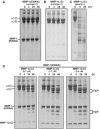
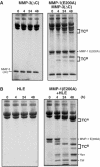
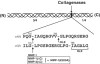
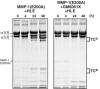
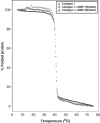
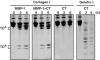
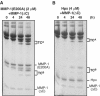
Breakdown of triple-helical interstitial collagens is essential in embryonic development, organ morphogenesis and tissue remodelling and repair. Aberrant collagenolysis may result in diseases such as arthritis, cancer, atherosclerosis, aneurysm and fibrosis. In vertebrates, it is initiated by collagenases belonging to the matrix metalloproteinase (MMP) family. The three-dimensional structure of a prototypic collagenase, MMP-1, indicates that the substrate-binding site of the enzyme is too narrow to accommodate triple-helical collagen. Here we report that collagenases bind and locally unwind the triple-helical structure before hydrolyzing the peptide bonds. Mutation of the catalytically essential residue Glu200 of MMP-1 to Ala resulted in a catalytically inactive enzyme, but in its presence noncollagenolytic proteinases digested collagen into typical 3/4 and 1/4 fragments, indicating that the MMP-1(E200A) mutant unwinds the triple-helical collagen. The study also shows that MMP-1 preferentially interacts with the alpha2(I) chain of type I collagen and cleaves the three alpha chains in succession. Our results throw light on the basic mechanisms that control a wide range of biological and pathological processes associated with tissue remodelling.
Figures


References
-
- Aimes RT, Quigley JP (1995) Matrix metalloproteinase-2 is an interstitial collagenase. Inhibitor-free enzyme catalyzes the cleavage of collagen fibrils and soluble native type I collagen generating the specific 3/4- and 1/4-length fragments. J Biol Chem 270: 5872–5876 - PubMed
-
- Bode W (1995) A helping hand for collagenases: the haemopexin-like domain. Structure 3: 527–530 - PubMed
-
- Brinckerhoff CE, Matrisian LM (2002) Matrix metalloproteinases: a tail of a frog that became a prince. Nat Rev Mol Cell Biol 3: 207–214 - PubMed
-
- Brown RA, Hukins DW, Weiss JB, Twose TM (1977) Do mammalian collagenases and DNA restriction endonucleases share a similar mechanism for cleavage site recognition? Biochem Biophys Res Commun 74: 1102–1108 - PubMed
-
- Cawston TE (1996) Metalloproteinase inhibitors and the prevention of connective tissue breakdown. Pharmacol Therap 70: 163–182 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

