Human geminin promotes pre-RC formation and DNA replication by stabilizing CDT1 in mitosis
- PMID: 15257290
- PMCID: PMC514931
- DOI: 10.1038/sj.emboj.7600314
Human geminin promotes pre-RC formation and DNA replication by stabilizing CDT1 in mitosis
Abstract
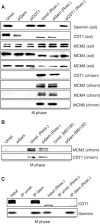
Geminin is an unstable inhibitor of DNA replication that negatively regulates the licensing factor CDT1 and inhibits pre-replicative complex (pre-RC) formation in Xenopus egg extracts. Here we describe a novel function of Geminin. We demonstrate that human Geminin protects CDT1 from proteasome-mediated degradation by inhibiting its ubiquitination. In particular, Geminin ensures basal levels of CDT1 during S phase and its accumulation during mitosis. Consistently, inhibition of Geminin synthesis during M phase leads to impairment of pre-RC formation and DNA replication during the following cell cycle. Moreover, we show that inhibition of CDK1 during mitosis, and not Geminin depletion, is sufficient for premature formation of pre-RCs, indicating that CDK activity is the major mitotic inhibitor of licensing in human cells. Taken together with recent data from our laboratory, our results demonstrate that Geminin is both a negative and positive regulator of pre-RC formation in human cells, playing a positive role in allowing CDT1 accumulation in G2-M, and preventing relicensing of origins in S-G2.
Figures






References
-
- Bielinsky AK (2003) Replication origins: why do we need so many? Cell Cycle 2: 307–309 - PubMed
-
- Cocker JH, Piatti S, Santocanale C, Nasmyth K, Diffley JF (1996) An essential role for the Cdc6 protein in forming the pre-replicative complexes of budding yeast. Nature 379: 180–182 - PubMed
-
- Dahmann C, Diffley JF, Nasmyth KA (1995) S-phase-promoting cyclin-dependent kinases prevent re-replication by inhibiting the transition of replication origins to a pre-replicative state. Curr Biol 5: 1257–1269 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

