Does the cannabinoid dronabinol reduce central pain in multiple sclerosis? Randomised double blind placebo controlled crossover trial
- PMID: 15258006
- PMCID: PMC498019
- DOI: 10.1136/bmj.38149.566979.AE
Does the cannabinoid dronabinol reduce central pain in multiple sclerosis? Randomised double blind placebo controlled crossover trial
Abstract
Objective: To evaluate the effect of the oral synthetic delta-9-tetrahydrocannabinol dronabinol on central neuropathic pain in patients with multiple sclerosis.
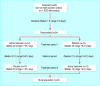
Design: Randomised double blind placebo controlled crossover trial.
Setting: Outpatient clinic, University Hospital of Aarhus, Denmark.
Participants: 24 patients aged between 23 and 55 years with multiple sclerosis and central pain.
Intervention: Orally administered dronabinol at a maximum dose of 10 mg daily or corresponding placebo for three weeks (15-21 days), separated by a three week washout period.
Main outcome measure: Median spontaneous pain intensity (numerical rating scale) in the last week of treatment.
Results: Median spontaneous pain intensity was significantly lower during dronabinol treatment than during placebo treatment (4.0 (25th to 75th centiles 2.3 to 6.0) v 5.0 (4.0 to 6.4), P = 0.02), and median pain relief score (numerical rating scale) was higher (3.0 (0 to 6.7) v> 0 (0 to 2.3), P = 0.035). The number needed to treat for 50% pain relief was 3.5 (95% confidence interval 1.9 to 24.8). On the SF-36 quality of life scale, the two items bodily pain and mental health indicated benefits from active treatment compared with placebo. The number of patients with adverse events was higher during active treatment, especially in the first week of treatment. The functional ability of the multiple sclerosis patients did not change.
Conclusions: Dronabinol has a modest but clinically relevant analgesic effect on central pain in patients with multiple sclerosis. Adverse events, including dizziness, were more frequent with dronabinol than with placebo during the first week of treatment.
Figures

Comment in
-
High hopes for cannabinoid analgesia.BMJ. 2004 Jul 31;329(7460):257-8. doi: 10.1136/bmj.38168.627292.0B. Epub 2004 Jul 16. BMJ. 2004. PMID: 15258005 Free PMC article. No abstract available.
References
-
- Archibald CJ, McGrath PJ, Ritvo PG, Fisk JD, Bhan V, Maxner CE, et al. Pain prevalence, severity and impact in a clinic sample of multiple sclerosis patients. Pain 1994;58: 89-93. - PubMed
-
- Clifford DB, Trotter JL. Pain in multiple sclerosis. Arch Neurol 1984;41: 1270-2. - PubMed
-
- Moulin DE, Foley KM, Ebers GC. Pain syndromes in multiple sclerosis. Neurology 1988;38: 1830-4. - PubMed
-
- Rae-Grant AD, Eckert NJ, Bartz S, Reed JF. Sensory symptoms of multiple sclerosis: a hidden reservoir of morbidity. Mult Scler 1999;5: 179-83. - PubMed
-
- Stenager E, Knudsen L, Jensen K. Acute and chronic pain syndromes in multiple sclerosis. Acta Neurol Scand 1991;84: 197-200. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
