Exocytosis of IgG as mediated by the receptor, FcRn: an analysis at the single-molecule level
- PMID: 15258288
- PMCID: PMC503743
- DOI: 10.1073/pnas.0402970101
Exocytosis of IgG as mediated by the receptor, FcRn: an analysis at the single-molecule level
Abstract
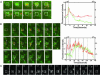
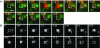
IgG transport within and across cells is essential for effective humoral immunity. Through a combination of biochemical and in vivo analyses, the MHC class I-related neonatal Fc receptor (FcRn) is known to play a central role in delivering IgGs within and across cells. However, little is known about the molecular and cellular mechanisms that are involved in the exocytosis of IgG from cells that express FcRn. Here, we use single-molecule fluorescence microscopy to analyze exocytic processes in FcRn-GFP-transfected human endothelial cells. We show that exocytosis can occur by means of multiple modes that range from complete fusion of the exocytic vesicle with the plasma membrane to a slower-release mode ("prolonged release") that only involves partial mixing of membrane contents. Even for prolonged release, diffusion of FcRn into the plasma membrane can occur, indicating that FcRn is directly involved in IgG exocytosis. The slower-release mode is characterized by periodic, stepwise release of IgG, rather than the rapid burst that is observed for complete-fusion events. Analyses of single-molecule tracks suggest that IgG may be bound to FcRn for several seconds after exocytosis. Unexpectedly, after diffusion out of the exocytic site, IgG and FcRn molecules can also migrate back into the epicenter of the release site. Such retrograde movement may represent a mechanism for FcRn retrieval. Our studies provide insight into the events that lead to IgG exocytosis.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

