Identification of retinal neurons in a regressive rodent eye (the naked mole-rat)
- PMID: 15259562
- PMCID: PMC1829152
- DOI: 10.1017/S0952523887043025
Identification of retinal neurons in a regressive rodent eye (the naked mole-rat)
Abstract
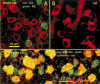
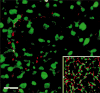
The retina consists of many parallel circuits designed to maximize the gathering of important information from the environment. Each of these circuits is comprised of a number of different cell types combined in modules that tile the retina. To a subterranean animal, vision is of relatively less importance. Knowledge of how circuits and their elements are altered in response to the subterranean environment is useful both in understanding processes of regressive evolution and in retinal processing itself. We examined common cell types in the retina of the naked mole-rat, Heterocephalus glaber with immunocytochemical markers and retrograde staining of ganglion cells from optic nerve injections. The stains used show that the naked mole-rat eye has retained multiple ganglion cell types, 1-2 types of horizontal cell, rod bipolar and multiple types of cone bipolar cells, and several types of common amacrine cells. However, no labeling was found with antibodies to the dopamine-synthesizing enzyme, tyrosine hydroxylase. Although most of the well-characterized mammalian cell types are present in the regressive mole-rat eye, their structural organization is considerably less regular than in more sighted mammals. We found less precision of depth of stratification in the inner plexiform layer and also less precision in their lateral coverage of the retina. The results suggest that image formation is not very important in these animals, but that circuits beyond those required for circadian entrainment remain in place.
Figures







Similar articles
-
Features of visual function in the naked mole-rat Heterocephalus glaber.J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2005 Apr;191(4):317-30. doi: 10.1007/s00359-004-0584-6. Epub 2005 Jan 13. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2005. PMID: 15647925
-
The eye of the african mole-rat Cryptomys anselli: to see or not to see?Eur J Neurosci. 2003 Feb;17(4):709-20. doi: 10.1046/j.1460-9568.2003.02485.x. Eur J Neurosci. 2003. PMID: 12603261
-
Central visual system of the naked mole-rat (Heterocephalus glaber).Anat Rec A Discov Mol Cell Evol Biol. 2006 Feb;288(2):205-12. doi: 10.1002/ar.a.20288. Anat Rec A Discov Mol Cell Evol Biol. 2006. PMID: 16419086
-
[Viral and Electrophysiological Approaches for Elucidating the Structure and Function of Retinal Circuits].Yakugaku Zasshi. 2018;138(5):669-678. doi: 10.1248/yakushi.17-00200-1. Yakugaku Zasshi. 2018. PMID: 29710012 Review. Japanese.
-
Inhibitory Interneurons in the Retina: Types, Circuitry, and Function.Annu Rev Vis Sci. 2017 Sep 15;3:1-24. doi: 10.1146/annurev-vision-102016-061345. Epub 2017 Jun 15. Annu Rev Vis Sci. 2017. PMID: 28617659 Review.
Cited by
-
Underdeveloped extraocular muscles in the naked mole-rat (Heterocephalus glaber).Anat Rec (Hoboken). 2010 May;293(5):918-23. doi: 10.1002/ar.21107. Anat Rec (Hoboken). 2010. PMID: 20186962 Free PMC article.
-
The naked truth: a comprehensive clarification and classification of current 'myths' in naked mole-rat biology.Biol Rev Camb Philos Soc. 2022 Feb;97(1):115-140. doi: 10.1111/brv.12791. Epub 2021 Sep 3. Biol Rev Camb Philos Soc. 2022. PMID: 34476892 Free PMC article. Review.
-
Compartmentation of the cerebellar cortex in the naked mole-rat (Heterocephalus glaber).Cerebellum. 2011 Sep;10(3):435-48. doi: 10.1007/s12311-011-0251-8. Cerebellum. 2011. PMID: 21298580
-
Distinct retinal ganglion cell types in strictly subterranean, naturally microphthalmic mammals.Proc Biol Sci. 2025 Jan;292(2038):20242586. doi: 10.1098/rspb.2024.2586. Epub 2025 Jan 15. Proc Biol Sci. 2025. PMID: 39809306
-
Regulation of arginase I activity and expression by both PD-1 and CTLA-4 on the myeloid-derived suppressor cells.Cancer Immunol Immunother. 2009 May;58(5):687-97. doi: 10.1007/s00262-008-0591-5. Epub 2008 Oct 1. Cancer Immunol Immunother. 2009. PMID: 18828017 Free PMC article.
References
-
- Baldridge WH, Ball AK. Background illumination reduces horizontal cell receptive-field size in both normal and 6-hydroxydopamine-lesioned goldfish retinas. Visual Neuroscience. 1991;7:441–450. - PubMed
-
- Bellesta J, Terenghi G, Thibault J, Polak JM. Putative dopamine-containing cells in the retina of seven species demonstrated by tyrosine hydroxylase immunocytochemistry. Neuroscience. 1984;12:1147–1156. - PubMed
-
- Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. - PubMed
-
- Boycott BB, Wässle H. Parallel processing in the mammalian retina: The Proctor lecture. Investigive Ophthalmology and Visual Science. 1999;40:1313–1327. - PubMed
-
- Brecha NC, Oyster CW, Takahashi ES. Identification and characterization of tyrosine hydroxylase immunoreactive amacrine cells. Investigative Ophthalmology and Visual Science. 1984;25:66–70. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

