Combinatorial Fgf and Bmp signalling patterns the gastrula ectoderm into prospective neural and epidermal domains
- PMID: 15262889
- PMCID: PMC2789263
- DOI: 10.1242/dev.01227
Combinatorial Fgf and Bmp signalling patterns the gastrula ectoderm into prospective neural and epidermal domains
Abstract
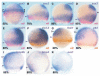
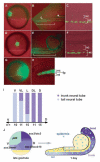
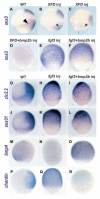
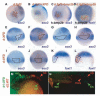
Studies in fish and amphibia have shown that graded Bmp signalling activity regulates dorsal-to-ventral (DV) patterning of the gastrula embryo. In the ectoderm, it is thought that high levels of Bmp activity promote epidermal development ventrally, whereas secreted Bmp antagonists emanating from the organiser induce neural tissue dorsally. However, in zebrafish embryos, the domain of cells destined to contribute to the spinal cord extends all the way to the ventral side of the gastrula, a long way from the organiser. We show that in vegetal (trunk and tail) regions of the zebrafish gastrula, neural specification is initiated at all DV positions of the ectoderm in a manner that is unaffected by levels of Bmp activity and independent of organiser-derived signals. Instead, we find that Fgf activity is required to induce vegetal prospective neural markers and can do so without suppressing Bmp activity. We further show that Bmp signalling does occur within the vegetal prospective neural domain and that Bmp activity promotes the adoption of caudal fate by this tissue.
Figures




References
-
- Agathon A, Thisse C, Thisse B. The molecular nature of the zebrafish tail organizer. Nature. 2003;424:448–452. - PubMed
-
- Akai J, Storey K. Brain or brawn: how FGF signaling gives us both. Cell. 2003;115:510–512. - PubMed
-
- Amacher SL, Draper BW, Summers BR, Kimmel CB. The zebrafish T-box genes no tail and spadetail are required for development of trunk and tail mesoderm and medial floor plate. Development. 2002;129:3311–3323. - PubMed
-
- Amaya E, Musci TJ, Kirschner MW. Expression of a dominant negative mutant of the FGF receptor disrupts mesoderm formation in Xenopus embryos. Cell. 1991;66:257–270. - PubMed
-
- Aybar MJ, Mayor R. Early induction of neural crest cells: lessons learned from frog, fish and chick. Curr. Opin. Genet. Dev. 2002;12:452–458. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

