Induction of a novel class of diacylglycerol acyltransferases and triacylglycerol accumulation in Mycobacterium tuberculosis as it goes into a dormancy-like state in culture
- PMID: 15262939
- PMCID: PMC451596
- DOI: 10.1128/JB.186.15.5017-5030.2004
Induction of a novel class of diacylglycerol acyltransferases and triacylglycerol accumulation in Mycobacterium tuberculosis as it goes into a dormancy-like state in culture
Abstract
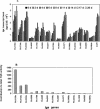
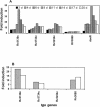
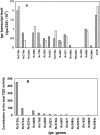
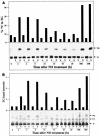
Mycobacterium tuberculosis enters the host by inhalation of an infectious aerosol and replicates in the alveolar macrophages until the host's immune defense causes bacteriostasis, which leads the pathogen to go into nonreplicative drug-resistant dormancy. The dormant pathogen can survive for decades till the host's immune system is weakened and active tuberculosis develops. Even though fatty acids are thought to be the major energy source required for the persistence phase, the source of fatty acids used is not known. We postulate that the pathogen uses triacylglycerol (TG) as a storage form of fatty acids. Little is known about the biosynthesis of TG in M. tuberculosis. We show that 15 mycobacterial genes that we identified as putative triacylglycerol synthase (tgs) when expressed in Escherichia coli showed TGS activity, and we report some basic catalytic characteristics of the most active enzymes. We show that several tgs genes are induced when the pathogen goes into the nonreplicative drug-resistant state caused by slow withdrawal of O(2) and also by NO treatment, which is known to induce dormancy-associated genes. The gene (Rv3130c) that shows the highest TGS activity when expressed in E. coli shows the highest induction by hypoxia and NO treatment. Biochemical evidence shows that TG synthesis and accumulation occur under both conditions. We conclude that TG may be a form of energy storage for use during long-term dormancy. Therefore, TG synthesis may be an appropriate target for novel antilatency drugs that can prevent the organism from surviving dormancy and thus assist in the control of tuberculosis.
Figures

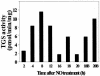
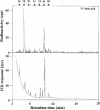
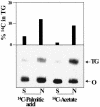
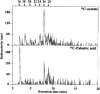
References
-
- Alvarez, H. M., and A. Steinbuchel. 2002. Triacylglycerols in prokaryotic microorganisms. Appl. Microbiol. Biotechnol. 60:367-376. - PubMed
-
- Asselineau, C., J. Asselineau, G. Laneelle, and M. A. Laneelle. 2002. The biosynthesis of mycolic acids by mycobacteria: current and alternative hypotheses. Prog. Lipid Res. 41:501-523. - PubMed
-
- Betts, J. C., P. T. Lukey, L. C. Robb, R. A. McAdam, and K. Duncan. 2002. Evaluation of a nutrient starvation model of Mycobacterium tuberculosis persistence by gene and protein expression profiling. Mol. Microbiol. 43:717-731. - PubMed
-
- Bishai, W. 2000. Lipid lunch for persistent pathogen. Nature 406:683-685. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

