Synaptotagmins are trafficked to distinct subcellular domains including the postsynaptic compartment
- PMID: 15263020
- PMCID: PMC2172321
- DOI: 10.1083/jcb.200312054
Synaptotagmins are trafficked to distinct subcellular domains including the postsynaptic compartment
Abstract
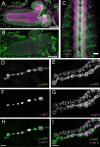
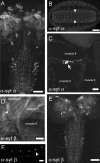
The synaptotagmin family has been implicated in calcium-dependent neurotransmitter release, although Synaptotagmin 1 is the only isoform demonstrated to control synaptic vesicle fusion. Here, we report the characterization of the six remaining synaptotagmin isoforms encoded in the Drosophila genome, including homologues of mammalian Synaptotagmins 4, 7, 12, and 14. Like Synaptotagmin 1, Synaptotagmin 4 is ubiquitously present at synapses, but localizes to the postsynaptic compartment. The remaining isoforms were not found at synapses (Synaptotagmin 7), expressed at very low levels (Synaptotagmins 12 and 14), or in subsets of putative neurosecretory cells (Synaptotagmins alpha and beta). Consistent with their distinct localizations, overexpression of Synaptotagmin 4 or 7 cannot functionally substitute for the loss of Synaptotagmin 1 in synaptic transmission. Our results indicate that synaptotagmins are differentially distributed to unique subcellular compartments. In addition, the identification of a postsynaptic synaptotagmin suggests calcium-dependent membrane-trafficking functions on both sides of the synapse.
Copyright The Rockerfeller University Press
Figures






References
-
- Adams, M.D., S.E. Celniker, R.A. Holt, C.A. Evans, J.D. Gocayne, P.G. Amanatides, S.E. Scherer, P.W. Li, R.A. Hoskins, R.F. Galle, et al. 2000. The genome sequence of Drosophila melanogaster. Science. 287:2185–2195. - PubMed
-
- Aparicio, S., J. Chapman, E. Stupka, N. Putnam, J.M. Chia, P. Dehal, A. Christoffels, S. Rash, S. Hoon, A. Smit, et al. 2002. Whole-genome shotgun assembly and analysis of the genome of Fugu rubripes. Science. 297:1301–1310. - PubMed
-
- Butz, S., R. Fernandez-Chacon, F. Schmitz, R. Jahn, and T.C. Südhof. 1999. The subcellular localizations of atypical synaptotagmins III and VI. Synaptotagmin III is enriched in synapses and synaptic plasma membranes but not in synaptic vesicles. J. Biol. Chem. 274:18290–18296. - PubMed
-
- Cantera, R., and D.R. Nassel. 1992. Segmental peptidergic innervation of abdominal targets in larval and adult dipteran insects revealed with an antiserum against leucokinin I. Cell Tissue Res. 269:459–471. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

