Therapeutic effects of PKC activators in Alzheimer's disease transgenic mice
- PMID: 15263077
- PMCID: PMC503753
- DOI: 10.1073/pnas.0403921101
Therapeutic effects of PKC activators in Alzheimer's disease transgenic mice
Abstract
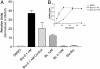
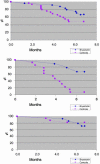
Alzheimer's disease (AD) characteristically presents with early memory loss. Regulation of K(+) channels, calcium homeostasis, and protein kinase C (PKC) activation are molecular events that have been implicated during associative memory which are also altered or defective in AD. PKC is also involved in the processing of the amyloid precursor protein (APP), a central element in AD pathophysiology. In previous studies, we demonstrated that benzolactam (BL), a novel PKC activator, reversed K(+) channels defects and enhanced secretion of APP alpha in AD cells. In this study we present data showing that another PKC activator, bryostatin 1, at subnanomolar concentrations dramatically enhances the secretion of the alpha-secretase product sAPP alpha in fibroblasts from AD patients. We also show that BL significantly increased the amount of sAPP alpha and reduced A beta 40 in the brains of APP[V717I] transgenic mice. In a more recently developed AD double-transgenic mouse, bryostatin was effective in reducing both brain A beta 40 and A beta 42. In addition, bryostatin ameliorated the rate of premature death and improved behavioral outcomes. Collectively, these data corroborate PKC and its activation as a potentially important means of ameliorating AD pathophysiology and perhaps cognitive impairment, thus offering a promising target for drug development. Because bryostatin 1 is devoid of tumor-promoting activity and is undergoing numerous clinical studies for cancer treatment in humans, it might be readily tested in patients as a potential therapeutic agent for Alzheimer's disease.
Figures



References
-
- Petersen, R. C., Smith, G. E., Invik, R. J., Kokmen, E. & Tangalos, E. G. (1994) Neurology 44, 867–872. - PubMed
-
- Bondi, M. W., Salmon, D. P. & Butters, N. (1994) in Alzheimer Disease, eds. Terry, R. D., Katzman, R. & Bick, K. L. (Raven Press, New York.), pp. 41–63.
-
- Alkon, D. L. (1984) Science 226, 1037–1045. - PubMed
-
- Alkon, D. L, Nelson, T. J, Zhao, W. & Cavallaro, S. (1998) Trends Neurosci. 21, 529–537. - PubMed
-
- Etcheberrigaray, R., Ito, E., Kim, C. S. & Alkon, D. L. (1994) Science 264, 276–279. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

