Irinotecan plus folinic acid/continuous 5-fluorouracil as simplified bimonthly FOLFIRI regimen for first-line therapy of metastatic colorectal cancer
- PMID: 15265233
- PMCID: PMC497044
- DOI: 10.1186/1471-2407-4-38
Irinotecan plus folinic acid/continuous 5-fluorouracil as simplified bimonthly FOLFIRI regimen for first-line therapy of metastatic colorectal cancer
Abstract
Background: Combination therapy of irinotecan, folinic acid (FA) and 5-fluorouracil (5-FU) has been proven to be highly effective for the treatment of metastatic colorectal cancer. However, in light of safety and efficacy concerns, the best combination regimen for first-line therapy still needs to be defined. The current study reports on the bimonthly FOLFIRI protocol consisting of irinotecan with continuous FA/5-FU in five German outpatient clinics, with emphasis on the safety and efficiency, quality of life, management of delayed diarrhea, and secondary resection of regressive liver metastases.
Methods: A total of 35 patients were treated for metastatic colorectal cancer. All patients received first-line treatment according to the FOLFIRI regimen, consisting of irinotecan (180 mg/m2), L-FA (200 mg/m2) and 5-FU bolus (400 mg/m2) on day 1, followed by a 46-h continuous infusion 5-FU (2400 mg/m2). One cycle contained three fortnightly administrations. Staging was performed after 2 cycles. Dosage was reduced at any time if toxicity NCI CTC grade III/IV was observed. Chemotherapy was administered only to diarrhea-free patients.
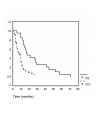
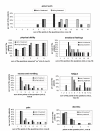
Results: The FOLFIRI regimen was generally well tolerated. It was postponed for one-week in 51 of 415 applications (12.3%). Dose reduction was necessary in ten patients. Grade III/IV toxicity was rare, with diarrhea (14%), nausea/vomiting (12%), leucopenia (3%), neutropenia (9%) and mucositis (3%). The overall response rate was 31% (4 CR and 7 PR), with disease control in 74%. After primary chemotherapy, resection of liver metastases was achieved in three patients. In one patient, the CR was confirmed pathologically. Median progression-free and overall survival were seven and 17 months, respectively.
Conclusions: The FOLFIRI regimen proved to be safe and efficient. Outpatient treatment was well tolerated. Since downstaging was possible, combinations of irinotecan and continuous FA/5-FU should further be investigated in neoadjuvant protocols.
Figures
Similar articles
-
Irinotecan plus S-1 (IRIS) versus fluorouracil and folinic acid plus irinotecan (FOLFIRI) as second-line chemotherapy for metastatic colorectal cancer: a randomised phase 2/3 non-inferiority study (FIRIS study).Lancet Oncol. 2010 Sep;11(9):853-60. doi: 10.1016/S1470-2045(10)70181-9. Epub 2010 Aug 12. Lancet Oncol. 2010. PMID: 20708966 Clinical Trial.
-
Efficacy and safety of an irinotecan plus bolus 5-fluorouracil and L-leucovorin regimen for metastatic colorectal cancer in Japanese patients: experience in a single institution in Japan.Jpn J Clin Oncol. 2007 Sep;37(9):686-91. doi: 10.1093/jjco/hym091. Epub 2007 Aug 24. Jpn J Clin Oncol. 2007. PMID: 17720736
-
Use of the folinic acid/5-fluorouracil/irinotecan (FOLFIRI 1) regimen in elderly patients as a first-line treatment for metastatic colorectal cancer: a Phase II study.Cancer Chemother Pharmacol. 2008 Nov;62(6):931-6. doi: 10.1007/s00280-008-0681-2. Epub 2008 Feb 14. Cancer Chemother Pharmacol. 2008. PMID: 18273618 Clinical Trial.
-
[Recent results of irinotecan therapy in colorectal cancer].Magy Onkol. 2004;48(4):281-8. Epub 2005 Jan 17. Magy Onkol. 2004. PMID: 15655572 Review. Hungarian.
-
Single-agent irinotecan or FOLFIRI as second-line chemotherapy for advanced colorectal cancer; results of a randomised phase II study (DaVINCI) and meta-analysis [corrected].Eur J Cancer. 2011 Aug;47(12):1826-36. doi: 10.1016/j.ejca.2011.04.024. Epub 2011 Jun 12. Eur J Cancer. 2011. PMID: 21665462 Clinical Trial.
Cited by
-
The prognostic values of EGFR expression and KRAS mutation in patients with synchronous or metachronous metastatic colorectal cancer.BMC Cancer. 2013 Dec 13;13:599. doi: 10.1186/1471-2407-13-599. BMC Cancer. 2013. PMID: 24330663 Free PMC article.
-
Capecitabine and irinotecan with and without bevacizumab for advanced colorectal cancer patients.World J Gastroenterol. 2009 Jan 28;15(4):449-56. doi: 10.3748/wjg.15.449. World J Gastroenterol. 2009. PMID: 19152449 Free PMC article. Clinical Trial.
-
Liposomal Irinotecan for Treatment of Colorectal Cancer in a Preclinical Model.Cancers (Basel). 2019 Feb 27;11(3):281. doi: 10.3390/cancers11030281. Cancers (Basel). 2019. PMID: 30818855 Free PMC article.
-
Irinotecan, continuous 5-fluorouracil, and low dose of leucovorin (modified FOLFIRI) as first line of therapy in recurrent or metastatic colorectal cancer.Korean J Intern Med. 2005 Sep;20(3):205-9. doi: 10.3904/kjim.2005.20.3.205. Korean J Intern Med. 2005. PMID: 16295778 Free PMC article.
-
Colorectal Cancer: Risk Factors, Novel Approaches in Molecular Screening and Treatment.Int J Mol Cell Med. 2025;14(1):576-605. doi: 10.22088/IJMCM.BUMS.14.1.576. Int J Mol Cell Med. 2025. PMID: 40123590 Free PMC article. Review.
References
-
- Modulation of fluorouracil by leucovorin in patients with advanced colorectal cancer: evidence in terms of response rate. Advanced Colorectal Cancer Meta-Analysis Project. J Clin Oncol. 1992;10:896–903. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

