Cisplatin-induced apoptosis in human malignant testicular germ cell lines depends on MEK/ERK activation
- PMID: 15266324
- PMCID: PMC2409982
- DOI: 10.1038/sj.bjc.6601919
Cisplatin-induced apoptosis in human malignant testicular germ cell lines depends on MEK/ERK activation
Abstract
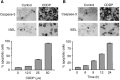
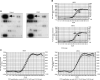
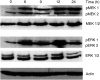
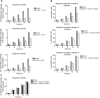
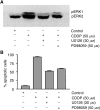
Testicular germ cell tumours (TGCT) represent the most common malignancies in young males. Whereas in 1970s, the survival rate in patients with metastatic testicular tumours was only 5%, these days, 80% of the patients treated by modern chemotherapy will survive their disease. The drug that revolutionised the cure rate for patients with metastatic testicular tumours was cisdiamminedichloroplatinum (cisplatin, CDDP). In vitro experiments on neoplastic germ cell lines showed that their exquisite sensitivity to CDDP could be attributed to p53-dependent and -independent pathways. Applying cDNA macroarray, semiquantitative RT-PCR and Western blot analyses, blocking experiments, caspase activity assays, and morphological methods, we sought here to define the p53-independent pathway(s) involved in the CDDP-induced apoptosis. For this purpose, we used the human TGCT cell line NCCIT, the mutated p53 of which is known to remain inactive during the course of CDDP-induced apoptosis. Our experiments showed that within hours of CDDP application, two prototype members of the 'mitogen-activated protein kinase' (MAPK) family, designated 'MAPK ERK kinase' (MEK) and 'extracellular signal-regulated kinase' (ERK), were dually phosphorylated and caspase-3 became active. Functional assays using MEK inhibitors demonstrated that the phosphorylation of MEK and ERK was required for the activation of caspase-3 as the executing caspase. Interestingly, experiments with the human malignant germ cell line NTERA, which is known to possess wild-type p53, revealed the same results. Thus, our data suggest that CDDP mediates its p53-independent apoptosis-inducing effect on the malignant human testicular germ cells--at least partially--through activation of the MEK-ERK signalling pathway. July 2004
Figures


Comment in
-
Cisplatin-induced apoptosis in human malignant testicular germ cell lines depends on MEK/ERK activation.J Urol. 2005 Aug;174(2):570-1. doi: 10.1016/s0022-5347(01)68314-9. J Urol. 2005. PMID: 16006899 No abstract available.
Similar articles
-
The role of reactive oxygen species in cisplatin-induced apoptosis in human malignant testicular germ cell lines.Int J Oncol. 2004 Dec;25(6):1671-6. doi: 10.3892/ijo.25.6.1671. Int J Oncol. 2004. PMID: 15547704
-
Expression and function of protein phosphatase PP2A in malignant testicular germ cell tumours.J Pathol. 2007 Sep;213(1):72-81. doi: 10.1002/path.2203. J Pathol. 2007. PMID: 17590861
-
The PDGFRβ-AKT pathway contributes to CDDP-acquired resistance in testicular germ cell tumors.Clin Cancer Res. 2014 Feb 1;20(3):658-67. doi: 10.1158/1078-0432.CCR-13-1131. Epub 2013 Nov 25. Clin Cancer Res. 2014. PMID: 24277456
-
The attractive Achilles heel of germ cell tumours: an inherent sensitivity to apoptosis-inducing stimuli.J Pathol. 2003 Jun;200(2):137-48. doi: 10.1002/path.1373. J Pathol. 2003. PMID: 12754734 Review.
-
Testicular germ cell tumours: the paradigm of chemo-sensitive solid tumours.Int J Biochem Cell Biol. 2005 Dec;37(12):2437-56. doi: 10.1016/j.biocel.2005.06.014. Epub 2005 Aug 11. Int J Biochem Cell Biol. 2005. PMID: 16099193 Review.
Cited by
-
Mitogen-activated protein kinase inhibition enhances the antitumor effects of sporamin in human pancreatic cancer cells.Oncol Lett. 2018 Jul;16(1):1237-1242. doi: 10.3892/ol.2018.8746. Epub 2018 May 18. Oncol Lett. 2018. PMID: 30061945 Free PMC article.
-
Role of N-cadherin in proliferation, migration, and invasion of germ cell tumours.Oncotarget. 2015 Oct 20;6(32):33426-37. doi: 10.18632/oncotarget.5288. Oncotarget. 2015. PMID: 26451610 Free PMC article.
-
The chemopreventive agent curcumin is a potent radiosensitizer of human cervical tumor cells via increased reactive oxygen species production and overactivation of the mitogen-activated protein kinase pathway.Mol Pharmacol. 2008 May;73(5):1491-501. doi: 10.1124/mol.107.043554. Epub 2008 Feb 5. Mol Pharmacol. 2008. PMID: 18252805 Free PMC article.
-
Benzo(a)pyrene Induced p53 Mediated Male Germ Cell Apoptosis: Synergistic Protective Effects of Curcumin and Resveratrol.Front Pharmacol. 2016 Aug 8;7:245. doi: 10.3389/fphar.2016.00245. eCollection 2016. Front Pharmacol. 2016. PMID: 27551266 Free PMC article.
-
C-Jun N-terminal kinase signalling pathway in response to cisplatin.J Cell Mol Med. 2016 Nov;20(11):2013-2019. doi: 10.1111/jcmm.12908. Epub 2016 Jul 4. J Cell Mol Med. 2016. PMID: 27374471 Free PMC article. Review.
References
-
- Bach BA, Knape WA, Edinger MG, Tubbs RR (1991) Improved sensitivity and resolution in the flow cytometric DNA analysis of human solid tumor specimens. Use of in vitro fine-needle aspiration and uniform staining reagents. Am J Clin Pathol 96: 615–627 - PubMed
-
- Boldt S, Widle UH, Kolch W (2002) The role of MAPK pathways in the action of chemotherapeutic drugs. Carcinogenesis 23: 1831–1838 - PubMed
-
- Burger H, Nooter K, Boersma AW, Kortland CJ, Stoter G (1997) Lack of correlation between cisplatin-induced apoptosis, p53 status and expression of Bcl-2 family proteins in testicular germ cell tumour cell lines. Int J Cancer 73: 592–599 - PubMed
-
- Burger H, Nooter K, Boersma AW, van Wingerden KE, Looijenga LH, Jochemsen AG, Stoter G (1999) Distinct p53-independent apoptotic cell death signalling pathways in testicular germ cell tumour cell lines. Int J Cancer 81: 620–628 - PubMed
-
- Chresta CM, Masters JRW, Hickman JA (1996) Hypersensitivity of human testicular tumors to etoposide-induced apoptosis is associated with functional p53 and a high Bax : Bcl-2 ratio. Cancer Res 56: 1834–1841 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

