Alteration of neuronal firing properties after in vivo experience in a FosGFP transgenic mouse
- PMID: 15269256
- PMCID: PMC6729874
- DOI: 10.1523/JNEUROSCI.4737-03.2004
Alteration of neuronal firing properties after in vivo experience in a FosGFP transgenic mouse
Abstract
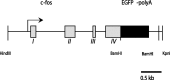
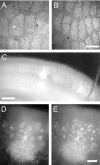
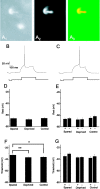
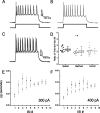
Identifying the cells and circuits that underlie perception, behavior, and learning is a central goal of contemporary neuroscience. Although techniques such as lesion analysis, functional magnetic resonance imaging, 2-deoxyglucose studies, and induction of gene expression have been helpful in determining the brain areas responsible for particular functions, these methods are technically limited. Currently, there is no method that allows for the identification and electrophysiological characterization of individual neurons that are associated with a particular function in living tissue. We developed a strain of transgenic mice in which the expression of the green fluorescent protein (GFP) is controlled by the promoter of the activity-dependent gene c-fos. These mice enable an in vivo or ex vivo characterization of the cells and synapses that are activated by particular pharmacological and behavioral manipulations. Cortical and subcortical fosGFP expression could be induced in a regionally restricted manner after specific activation of neuronal ensembles. Using the fosGFP mice to identify discrete cortical areas, we found that neurons in sensory-spared areas rapidly regulate action potential threshold and spike frequency to decrease excitability. This method will enhance our ability to study the way neuronal networks are activated and changed by both experience and pharmacological manipulations. In addition, because activated neurons can be functionally characterized, this tool may enable the development of better pharmaceuticals that directly affect the neurons involved in disease states.
Figures




References
-
- Barth AL (2002) Differential plasticity in neocortical networks. Physiol Behav 77: 545-550. - PubMed
-
- Bernstein LR, Ferris DK, Colburn NH, Sobel ME (1994) A family of mitogen-activated protein kinase-related proteins interacts in vivo with activator protein-1 transcription factor. J Biol Chem 269: 9401-9404. - PubMed
-
- Cole AJ, Saffen DW, Baraban JM, Worley PF (1989) Rapid increase of an immediate early gene messenger RNA in hippocampal neurons by synaptic NMDA receptor activation. Nature 340: 474-476. - PubMed
-
- Curran T (1988) The fos oncogene. In: The oncogene handbook (Reddy EP, Skalka AM, Curran T, eds), pp 307-325. Amsterdam: Elsevier Science.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
