Modulation of synaptic plasticity by antimanic agents: the role of AMPA glutamate receptor subunit 1 synaptic expression
- PMID: 15269270
- PMCID: PMC6729868
- DOI: 10.1523/JNEUROSCI.1258-04.2004
Modulation of synaptic plasticity by antimanic agents: the role of AMPA glutamate receptor subunit 1 synaptic expression
Abstract
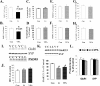
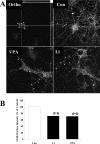
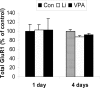
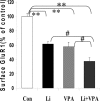
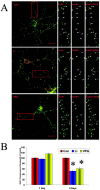
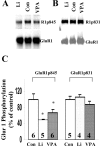
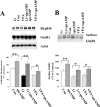
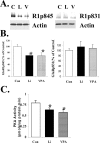
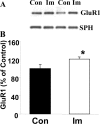
Increasing data suggest that impairments of cellular plasticity underlie the pathophysiology of bipolar disorder. In this context, it is noteworthy that AMPA glutamate receptor trafficking regulates synaptic plasticity, effects mediated by signaling cascades, which are targets for antimanic agents. The present studies were undertaken to determine whether two clinically effective, but structurally highly dissimilar, antimanic agents lithium and valproate regulate synaptic expression of AMPA receptor subunit glutamate receptor 1 (GluR1). Chronic (but not acute) treatment of rats with therapeutically relevant concentrations of lithium or valproate reduced hippocampal synaptosomal GluR1 levels. The reduction in synaptic GluR1 by lithium and valproate was attributable to a reduction of surface GluR1 distribution onto the neuronal membrane as demonstrated by three independent assays in cultured hippocampal neurons. Furthermore, these agents induced a decrease in GluR1 phosphorylation at a specific PKA site (GluR1p845), which is known to be critical for AMPA receptor insertion. Sp-cAMP treatment reversed the attenuation of phosphorylation by lithium and valproate and also brought GluR1 back to the surface, suggesting that phosphorylation of GluR1p845 is involved in the mechanism of GluR1 surface attenuation. In addition, GluR1p845 phosphorylation also was attenuated in hippocampus from lithium- or valproate-treated animals in vivo. In contrast, imipramine, an antidepressant that can trigger manic episodes, increased synaptic expression of GluR1 in hippocampus in vivo. These studies suggest that regulation of glutamatergically mediated synaptic plasticity may play a role in the treatment of bipolar disorder and raise the possibility that agents more directly affecting synaptic GluR1 may represent novel therapies for this devastating illness.
Figures

References
-
- Backstrom P, Hyytia P (2003) Attenuation of cocaine-seeking behaviour by the AMPA/kainate receptor antagonist CNQX in rats. Psychopharmacology (Berl) 166: 69-76. - PubMed
-
- Burns LH, Everitt BJ, Kelley AE, Robbins TW (1994) Glutamate-dopamine interactions in the ventral striatum: role in locomotor activity and responding with conditioned reinforcement. Psychopharmacology (Berl) 115: 516-528. - PubMed
-
- Carlezon Jr WA, Nestler EJ (2002) Elevated levels of GluR1 in the midbrain: a trigger for sensitization to drugs of abuse? Trends Neurosci 25: 610-615. - PubMed
-
- Chao SZ, Ariano MA, Peterson DA, Wolf ME (2002) D1 dopamine receptor stimulation increases GluR1 surface expression in nucleus accumbens neurons. J Neurochem 83: 704-712. - PubMed
-
- Coyle JT, Duman RS (2003) Finding the intracellular signaling pathways affected by mood disorder treatments. Neuron 38: 157-160. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
