Cellular and genetic analysis of wound healing in Drosophila larvae
- PMID: 15269788
- PMCID: PMC479041
- DOI: 10.1371/journal.pbio.0020239
Cellular and genetic analysis of wound healing in Drosophila larvae
Abstract
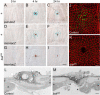
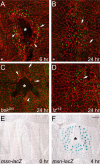
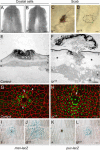
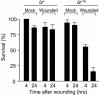
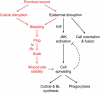
To establish a genetic system to study postembryonic wound healing, we characterized epidermal wound healing in Drosophila larvae. Following puncture wounding, larvae begin to bleed but within an hour a plug forms in the wound gap. Over the next couple of hours the outer part of the plug melanizes to form a scab, and epidermal cells surrounding the plug orient toward it and then fuse to form a syncytium. Subsequently, more-peripheral cells orient toward and fuse with the central syncytium. During this time, the Jun N-terminal kinase (JNK) pathway is activated in a gradient emanating out from the wound, and the epidermal cells spread along or through the wound plug to reestablish a continuous epithelium and its basal lamina and apical cuticle lining. Inactivation of the JNK pathway inhibits epidermal spreading and reepithelialization but does not affect scab formation or other wound healing responses. Conversely, mutations that block scab formation, and a scabless wounding procedure, provide evidence that the scab stabilizes the wound site but is not required to initiate other wound responses. However, in the absence of a scab, the JNK pathway is hyperinduced, reepithelialization initiates but is not always completed, and a chronic wound ensues. The results demonstrate that the cellular responses of wound healing are under separate genetic control, and that the responses are coordinated by multiple signals emanating from the wound site, including a negative feedback signal between scab formation and the JNK pathway. Cell biological and molecular parallels to vertebrate wound healing lead us to speculate that wound healing is an ancient response that has diversified during evolution.
Conflict of interest statement
The authors have declared that no conflicts of interest exist.
Figures



References
-
- Alster TS, Tanzi EL. Hypertrophic scars and keloids: Etiology and management. Am J Clin Dermatol. 2003;4:235–243. - PubMed
-
- Ashcroft GS, Yang X, Glick AB, Weinstein M, Letterio JL, et al. Mice lacking Smad3 show accelerated wound healing and an impaired local inflammatory response. Nat Cell Biol. 1999;1:260–266. - PubMed
-
- Barwig B. Isolation and characterization of plasma coagulogen of the cockroach Leucophaea maderae (Blattaria) J Comp Physiol B. 1985;155:135–143.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

