MAPKKKalpha is a positive regulator of cell death associated with both plant immunity and disease
- PMID: 15272302
- PMCID: PMC514913
- DOI: 10.1038/sj.emboj.7600283
MAPKKKalpha is a positive regulator of cell death associated with both plant immunity and disease
Abstract
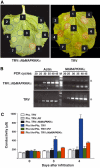
Many plant pathogens cause disease symptoms that manifest over days as regions of localized cell death. Localized cell death (the hypersensitive response; HR) also occurs in disease-resistant plants, but this response appears within hours of attempted infection and may restrict further pathogen growth. We identified a MAP kinase kinase kinase gene (MAPKKKalpha) that is required for the HR and resistance against Pseudomonas syringae. Significantly, we found that MAPKKKalpha also regulates cell death in susceptible leaves undergoing P. syringae infection. Overexpression of MAPKKKalpha in leaves activated MAPKs and caused pathogen-independent cell death. By overexpressing MAPKKKalpha in leaves and suppressing expression of various MAPKK and MAPK genes by virus-induced gene silencing, we identified two distinct MAPK cascades that act downstream of MAPKKKalpha. These results demonstrate that signal transduction pathways associated with both plant immunity and disease susceptibility share a common molecular switch.
Figures






References
-
- Asai T, Tena G, Plotnikova J, Willmann MR, Chiu W-L, Gomez-Gomez L, Boller T, Ausubel FM, Sheen J (2002) MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 415: 977–983 - PubMed
-
- del Pozo O, Lam E (2003) Expression of the baculovirus p35 protein in tobacco affects cell death progression and compromises N gene-mediated disease resistance response to Tobacco mosaic virus. Mol Plant Microbe Interact 16: 485–494 - PubMed
-
- Ekengren SK, Liu Y, Schiff M, Dinesh-Kumar SP, Martin GB (2003) Two MAPK cascades, NPR1, and TGA transcription factors play a role in Pto-mediated disease resistance in tomato. Plant J 36: 905–917 - PubMed
-
- Goodman RN, Novacky AJ (1994) The Hypersensitive Reaction in Plants to Pathogens: A Resistance Phenomenon. St Paul, MN: The American Phytopathological Society
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

