CD95 ligand induces motility and invasiveness of apoptosis-resistant tumor cells
- PMID: 15272306
- PMCID: PMC514938
- DOI: 10.1038/sj.emboj.7600325
CD95 ligand induces motility and invasiveness of apoptosis-resistant tumor cells
Abstract
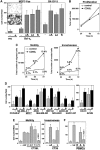
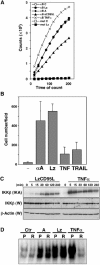
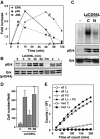
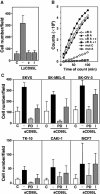
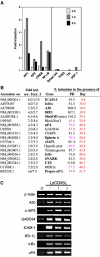
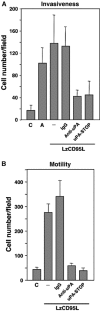
The apoptosis-inducing death receptor CD95 (APO-1/Fas) controls the homeostasis of many tissues. Despite its apoptotic potential, most human tumors are refractory to the cytotoxic effects of CD95 ligand. We now show that CD95 stimulation of multiple apoptosis-resistant tumor cells by CD95 ligand induces increased motility and invasiveness, a response much less efficiently triggered by TNFalpha or TRAIL. Three signaling pathways resulting in activation of NF-kappaB, Erk1/2 and caspase-8 were found to be important to this novel activity of CD95. Gene chip analyses of a CD95-stimulated tumor cell line identified a number of potential survival genes and genes that are known to regulate increased motility and invasiveness of tumor cells to be induced. Among these genes, urokinase plasminogen activator was found to be required for the CD95 ligand-induced motility and invasiveness. Our data suggest that CD95L, which is found elevated in many human cancer patients, has tumorigenic activities on human cancer cells. This could become highly relevant during chemotherapy, which can cause upregulation of CD95 ligand by both tumor and nontumor cells.
Figures

References
-
- Ahn JH, Park SM, Cho HS, Lee MS, Yoon JB, Vilcek J, Lee TH (2001) Non-apoptotic signaling pathways activated by soluble Fas ligand in serum-starved human fibroblasts. Mitogen-activated protein kinases and NF-kappaB-dependent gene expression. J Biol Chem 276: 47100–47106 - PubMed
-
- Aoki K, Kurooka M, Chen JJ, Petryniak J, Nabel EG, Nabel GJ (2001) Extracellular matrix interacts with soluble CD95L: retention and enhancement of cytotoxicity. Nat Immunol 2: 333–337 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

