Xenopus paraxial protocadherin has signaling functions and is involved in tissue separation
- PMID: 15272309
- PMCID: PMC514504
- DOI: 10.1038/sj.emboj.7600329
Xenopus paraxial protocadherin has signaling functions and is involved in tissue separation
Abstract
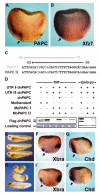
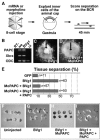
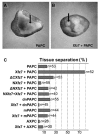
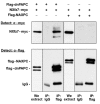
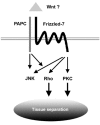
Protocadherins have homophilic adhesion properties and mediate selective cell-cell adhesion and cell sorting. Knockdown of paraxial protocadherin (PAPC) function in the Xenopus embryo impairs tissue separation, a process that regulates separation of cells of ectodermal and mesodermal origin during gastrulation. We show that PAPC can modulate the activity of the Rho GTPase and c-jun N-terminal kinase, two regulators of the cytoskeletal architecture and effectors of the planar cell polarity pathway. This novel signaling function of PAPC is essential for the regulation of tissue separation. In addition, PAPC can interact with the Xenopus Frizzled 7 receptor, and both proteins contribute to the development of separation behavior by activating Rho and protein kinase Calpha.
Figures



Similar articles
-
Paraxial protocadherin mediates cell sorting and tissue morphogenesis by regulating C-cadherin adhesion activity.J Cell Biol. 2006 Jul 17;174(2):301-13. doi: 10.1083/jcb.200602062. J Cell Biol. 2006. PMID: 16847104 Free PMC article.
-
Paraxial protocadherin coordinates cell polarity during convergent extension via Rho A and JNK.EMBO J. 2004 Aug 18;23(16):3259-69. doi: 10.1038/sj.emboj.7600332. Epub 2004 Aug 5. EMBO J. 2004. PMID: 15297873 Free PMC article.
-
PAPC and the Wnt5a/Ror2 pathway control the invagination of the otic placode in Xenopus.BMC Dev Biol. 2011 Jun 10;11:36. doi: 10.1186/1471-213X-11-36. BMC Dev Biol. 2011. PMID: 21663658 Free PMC article.
-
Cell migration in the Xenopus gastrula.Wiley Interdiscip Rev Dev Biol. 2018 Nov;7(6):e325. doi: 10.1002/wdev.325. Epub 2018 Jun 26. Wiley Interdiscip Rev Dev Biol. 2018. PMID: 29944210 Review.
-
Forces driving cell sorting in the amphibian embryo.Mech Dev. 2017 Apr;144(Pt A):81-91. doi: 10.1016/j.mod.2016.09.003. Epub 2016 Sep 30. Mech Dev. 2017. PMID: 27697520 Review.
Cited by
-
Deciphering animal development through proteomics: requirements and prospects.Proteome Sci. 2008 Jul 24;6:21. doi: 10.1186/1477-5956-6-21. Proteome Sci. 2008. PMID: 18652672 Free PMC article.
-
Cell-cell contact landscapes in Xenopus gastrula tissues.Proc Natl Acad Sci U S A. 2021 Sep 28;118(39):e2107953118. doi: 10.1073/pnas.2107953118. Proc Natl Acad Sci U S A. 2021. PMID: 34544871 Free PMC article.
-
Nuclear signaling from cadherin adhesion complexes.Curr Top Dev Biol. 2015;112:129-96. doi: 10.1016/bs.ctdb.2014.11.018. Epub 2015 Feb 12. Curr Top Dev Biol. 2015. PMID: 25733140 Free PMC article. Review.
-
A protocadherin-cadherin-FLRT3 complex controls cell adhesion and morphogenesis.PLoS One. 2009 Dec 22;4(12):e8411. doi: 10.1371/journal.pone.0008411. PLoS One. 2009. PMID: 20027292 Free PMC article.
-
Molecular basis of morphogenesis during vertebrate gastrulation.Cell Mol Life Sci. 2009 Jul;66(14):2263-73. doi: 10.1007/s00018-009-0018-2. Epub 2009 Apr 4. Cell Mol Life Sci. 2009. PMID: 19347571 Free PMC article. Review.
References
-
- Alagramam KN, Murcia CL, Kwon HY, Pawlowski KS, Wright CG, Woychik RP (2001a) The mouse Ames waltzer hearing-loss mutant is caused by mutation of Pcdh15, a novel protocadherin gene. Nat Genet 27: 99–102 - PubMed
-
- Alagramam KN, Yuan H, Kuehn MH, Murcia CL, Wayne S, Srisailpathy CR, Lowry RB, Knaus R, Van Laer L, Bernier FP, Schwartz S, Lee C, Morton CC, Mullins RF, Ramesh A, Van Camp G, Hageman GS, Woychik RP, Smith RJ, Hageman GS (2001b) Mutations in the novel protocadherin PCDH15 cause Usher syndrome type 1F. Hum Mol Genet 10: 1709–1718 - PubMed
-
- Bradley RS, Espeseth A, Kintner C (1998) NF-protocadherin, a novel member of the cadherin superfamily, is required for Xenopus ectodermal differentiation. Curr Biol 8: 325–334 - PubMed
-
- Clark HF, Brentrup D, Schneitz K, Bieber A, Goodman C, Noll M (1995) Dachsous encodes a member of the cadherin superfamily that controls imaginal disc morphogenesis in Drosophila. Genes Dev 9: 1530–1542 - PubMed
-
- Das G, Reynolds-Kenneally J, Mlodzik M (2002) The atypical cadherin Flamingo links Frizzled and Notch signaling in planar polarity establishment in the Drosophila eye. Dev Cell 2: 655–666 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

