Estimation of serum-free 50-percent inhibitory concentrations for human immunodeficiency virus protease inhibitors lopinavir and ritonavir
- PMID: 15273100
- PMCID: PMC478510
- DOI: 10.1128/AAC.48.8.2911-2917.2004
Estimation of serum-free 50-percent inhibitory concentrations for human immunodeficiency virus protease inhibitors lopinavir and ritonavir
Abstract
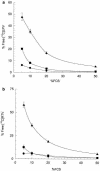
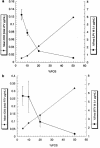
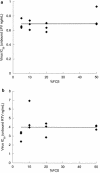
Using measured free fraction and 50% inhibitory concentration (IC50) values for the human immunodeficiency virus protease inhibitors lopinavir (LPV) and ritonavir (RTV) in tissue culture media with various protein concentrations ranging from 5 to 50%, we estimated serum-free IC50 values for each drug. The range of serum-free IC50 values (0.64 to 0.77 ng/ml for LPV and 3.0 to 5.0 ng/ml for RTV) did not exhibit a trend with increasing protein concentrations, despite a 10-fold difference in the free fraction value (0.006 to 0.063) for LPV and a 5-fold difference in the free fraction value (0.013 to 0.057) for RTV. The mean serum-free IC50 by the MTT-MT4 assay (0.69 ng/ml for LPV and 4.0 ng/ml for RTV) may be the most accurate parameter for the estimation of the inhibitory quotient (IQ), a relative measure of in vivo potency defined as the ratio of the minimal free drug concentration in plasma (C(trough,free)) for a specific patient population and the serum-free IC50. Using this approach, we calculated the average IQs for protease inhibitor-naïve patients for LPV and RTV to be 67 and 5.6, respectively.
Figures
Similar articles
-
Determination of lopinavir and ritonavir in blood plasma, seminal plasma, saliva and plasma ultra-filtrate by liquid chromatography/tandem mass spectrometry detection.Rapid Commun Mass Spectrom. 2008;22(5):657-64. doi: 10.1002/rcm.3411. Rapid Commun Mass Spectrom. 2008. PMID: 18257112
-
Ritonavir-boosted atazanavir-lopinavir combination: a pharmacokinetic interaction study of total, unbound plasma and cellular exposures.Antivir Ther. 2006;11(1):53-62. Antivir Ther. 2006. PMID: 16518960 Clinical Trial.
-
The use of drug resistance algorithms and genotypic inhibitory quotient in prediction of lopinavir-ritonavir treatment response in human immunodeficiency virus type 1 protease inhibitor-experienced patients.J Clin Virol. 2007 Feb;38(2):131-8. doi: 10.1016/j.jcv.2006.11.011. Epub 2007 Jan 4. J Clin Virol. 2007. PMID: 17208042
-
Prediction of early and confirmed virological response by genotypic inhibitory quotients for lopinavir in patients naïve for lopinavir with limited exposure to previous protease inhibitors.J Clin Virol. 2006 Apr;35(4):414-9. doi: 10.1016/j.jcv.2005.10.001. Epub 2005 Nov 8. J Clin Virol. 2006. PMID: 16280255 Clinical Trial.
-
Ability of different lopinavir genotypic inhibitory quotients to predict 48-week virological response in highly treatment-experienced HIV-infected patients receiving lopinavir/ritonavir.J Med Virol. 2006 Dec;78(12):1537-41. doi: 10.1002/jmv.20736. J Med Virol. 2006. PMID: 17063520
Cited by
-
Protein binding of lopinavir and ritonavir during 4 phases of pregnancy: implications for treatment guidelines.J Acquir Immune Defic Syndr. 2013 May 1;63(1):51-8. doi: 10.1097/QAI.0b013e31827fd47e. J Acquir Immune Defic Syndr. 2013. PMID: 23221983 Free PMC article.
-
Physiologically-Based Pharmacokinetic Modeling to Predict the Clinical Efficacy of the Coadministration of Lopinavir and Ritonavir against SARS-CoV-2.Clin Pharmacol Ther. 2020 Dec;108(6):1176-1184. doi: 10.1002/cpt.2014. Epub 2020 Oct 6. Clin Pharmacol Ther. 2020. PMID: 32767755 Free PMC article.
-
Deriving protein binding-corrected chemical concentrations for in vitro testing.Clin Transl Sci. 2023 Nov;16(11):2123-2129. doi: 10.1111/cts.13616. Epub 2023 Aug 28. Clin Transl Sci. 2023. PMID: 37605430 Free PMC article.
-
Model-Based Analysis of Unbound Lopinavir Pharmacokinetics in HIV-Infected Pregnant Women Supports Standard Dosing in the Third Trimester.CPT Pharmacometrics Syst Pharmacol. 2016 Mar;5(3):147-57. doi: 10.1002/psp4.12065. Epub 2016 Mar 22. CPT Pharmacometrics Syst Pharmacol. 2016. PMID: 27069778 Free PMC article.
-
Toward Consensus on Correct Interpretation of Protein Binding in Plasma and Other Biological Matrices for COVID-19 Therapeutic Development.Clin Pharmacol Ther. 2021 Jul;110(1):64-68. doi: 10.1002/cpt.2099. Epub 2020 Nov 21. Clin Pharmacol Ther. 2021. PMID: 33113246 Free PMC article. Review.
References
-
- Anderson, P. L., R. C. Brundage, L. Bushman, T. N. Kakuda, R. P. Remmel, and C. V. Fletcher. 2000. Indinavir plasma protein binding in HIV-1-infected adults. AIDS 14:2293-2297. - PubMed
-
- Becker, S., A. Fisher, C. Flexner, J. G. Gerber, R. Haubrich, A. D. M. Kashuba, A. D. Luber, and S. C. Piscitelli. 2001. Pharmacokinetic parameters of protease inhibitors and the Cmin/IC50 ratio: call for consensus. J. Acquir. Immune Defic. Syndr. 27:210-211. - PubMed
-
- Bilello, J. A., P. A. Bilello, M. Prichard, T. Robins, and G. L. Drusano. 1995. Reduction of the in vitro activity of A77003, an inhibitor of human immunodeficiency virus protease by human serum α1 acid glycoprotein. J. Infect. Dis. 171:546-551. - PubMed
-
- Bilello, J. A., P. A. Bilello, K. Stellrecht, J. Leonard, D. W. Norbeck, D. J. Kempf, T. Robins, and G. L. Drusano. 1996. Human serum α1 acid glycoprotein reduces uptake, intracellular concentration, and antiviral activity of A-80987, an inhibitor of the human immunodeficiency virus type-1 protease. Antimicrob. Agents Chemother. 40:1491-1497. - PMC - PubMed
-
- Bilello, J. A., and G. L. Drusano. 1996. Relevance of plasma protein binding to antiviral activity and clinical efficacy of inhibitors of human immunodeficiency virus protease. J. Infect. Dis. 173:1524-1525. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

