Nitric oxide generated from isoniazid activation by KatG: source of nitric oxide and activity against Mycobacterium tuberculosis
- PMID: 15273113
- PMCID: PMC478481
- DOI: 10.1128/AAC.48.8.3006-3009.2004
Nitric oxide generated from isoniazid activation by KatG: source of nitric oxide and activity against Mycobacterium tuberculosis
Abstract
Isonicotinic acid hydrazide (INH) is a frontline antituberculosis agent. Once taken up by Mycobacterium tuberculosis, INH requires activation by the catalase-peroxidase KatG, converting INH from its prodrug form into a range of bactericidal reactive species. Here we used 15N-labeled INH together with electron paramagnetic resonance spin trapping techniques to demonstrate that nitric oxide (NO*) is generated from oxidation at the hydrazide nitrogens during the activation of INH by M. tuberculosis KatG. We also observed that a specific scavenger of NO* provided protection against the antimycobacterial activity of INH in bacterial culture. No significant increases in mycobacterial protein nitration were detected, suggesting that NOdot; and not peroxynitrite, a nitrating metabolite of NO*, is involved in antimycobacterial action. In conclusion, INH-derived NO* has biological activity, which directly contributes to the antimycobacterial action of INH.
Figures

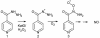
References
-
- Banerjee, A., E. Dubnau, A. Quemard, V. Balasubramanian, K. S. Um, T. Wilson, D. Collins, G. de Lisle, and W. R. Jacobs, Jr. 1994. inhA, a gene encoding a target for isoniazid and ethionamide in Mycobacterium tuberculosis. Science 263:227-230. - PubMed
-
- Beckmann, J. S., Y. Z. Ye, P. G. Anderson, J. Chen, M. A. Accavitti, M. M. Tarpey, and C. R. White. 1994. Extensive nitration of protein tyrosines in human atherosclerosis detected by immunohistochemistry. Biol. Chem. Hoppe-Seyler 375:81-88. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

