Possible mechanism of miltefosine-mediated death of Leishmania donovani
- PMID: 15273114
- PMCID: PMC478494
- DOI: 10.1128/AAC.48.8.3010-3015.2004
Possible mechanism of miltefosine-mediated death of Leishmania donovani
Abstract
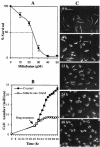
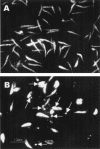
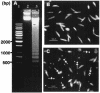
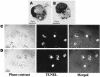
Miltefosine causes leishmanial death, but the possible mechanism(s) of action is not known. The mode of action of miltefosine was investigated in vitro in Leishmania donovani promastigotes as well as in extra- and intracellular amastigotes. Here, we demonstrate that miltefosine induces apoptosis-like death in L. donovani based on observed phenomena such as nuclear DNA condensation, DNA fragmentation with accompanying ladder formation, and in situ labeling of DNA fragments by the terminal deoxyribonucleotidyltransferase-mediated dUTP-biotin nick end labeling method. Understanding of miltefosine-mediated death will facilitate the design of new therapeutic strategies against Leishmania parasites.
Figures

Similar articles
-
15d-Prostaglandin J2 induced reactive oxygen species-mediated apoptosis during experimental visceral leishmaniasis.J Mol Med (Berl). 2016 Jun;94(6):695-710. doi: 10.1007/s00109-016-1384-5. Epub 2016 Feb 1. J Mol Med (Berl). 2016. PMID: 26830627
-
Miltefosine induces programmed cell death in Leishmania amazonensis promastigotes.Mem Inst Oswaldo Cruz. 2011 Jun;106(4):507-9. doi: 10.1590/s0074-02762011000400021. Mem Inst Oswaldo Cruz. 2011. PMID: 21739043
-
Miltefosine induces apoptosis in arsenite-resistant Leishmania donovani promastigotes through mitochondrial dysfunction.Exp Parasitol. 2007 May;116(1):1-13. doi: 10.1016/j.exppara.2006.10.007. Epub 2006 Dec 11. Exp Parasitol. 2007. PMID: 17161839
-
[Treatment of visceral leishmaniasis: efficacy and limits of miltefosine].Sante. 2001 Oct-Dec;11(4):257-8. Sante. 2001. PMID: 11861203 Review. French. No abstract available.
-
The preclinical discovery and development of oral miltefosine for the treatment of visceral leishmaniasis: a case history.Expert Opin Drug Discov. 2020 Jun;15(6):647-658. doi: 10.1080/17460441.2020.1743674. Epub 2020 Mar 23. Expert Opin Drug Discov. 2020. PMID: 32202449 Review.
Cited by
-
Alteration of fatty acid and sterol metabolism in miltefosine-resistant Leishmania donovani promastigotes and consequences for drug-membrane interactions.Antimicrob Agents Chemother. 2005 Jul;49(7):2677-86. doi: 10.1128/AAC.49.7.2677-2686.2005. Antimicrob Agents Chemother. 2005. PMID: 15980336 Free PMC article.
-
Activity and Cell-Death Pathway in Leishmania infantum Induced by Sugiol: Vectorization Using Yeast Cell Wall Particles Obtained From Saccharomyces cerevisiae.Front Cell Infect Microbiol. 2019 Jun 14;9:208. doi: 10.3389/fcimb.2019.00208. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31259161 Free PMC article.
-
Tolerance to drug-induced cell death favours the acquisition of multidrug resistance in Leishmania.Cell Death Dis. 2011 Sep 1;2(9):e201. doi: 10.1038/cddis.2011.83. Cell Death Dis. 2011. PMID: 21881603 Free PMC article.
-
Metacaspase-binding peptide inhibits heat shock-induced death in Leishmania (L.) amazonensis.Cell Death Dis. 2017 Mar 2;8(3):e2645. doi: 10.1038/cddis.2017.59. Cell Death Dis. 2017. PMID: 28252649 Free PMC article.
-
Miltefosine (hexadecylphosphocholine) inhibits cytochrome c oxidase in Leishmania donovani promastigotes.Antimicrob Agents Chemother. 2007 Apr;51(4):1327-32. doi: 10.1128/AAC.01415-06. Epub 2007 Feb 5. Antimicrob Agents Chemother. 2007. PMID: 17283192 Free PMC article.
References
-
- Ameisen, J. C. 1996. The origin of programmed cell death. Science 272:1278-1279. - PubMed
-
- Arnoult, D., K. Akarid, A. Grodet, P. X. Petit, J. Estaquier, and J. C. Ameisen. 2002. On the evaluation of programmed cell death: apoptosis of the unicellular eukaryote Leishmania major involves cysteine proteinase activation and mitochondrion permeabilization. Cell Death Differ. 9:65-81. - PubMed
-
- Arvind, L., V. M. Dixit, and E. V. Konin. 2001. Apoptotic molecular machinery: vastly increased complexity in vertebrates revealed by genome comparisons. Science 291:1279-1284. - PubMed
-
- Balanco, J. M. F., M. E. C. Moreira, A. Bonomo, P. T. Bozza, G. A. Mendes, C. Pirmez, and M. A. Barcinski. 2001. Apoptotic mimicry by an obligate intracellular parasite downregulates macrophage microbial activity. Curr. Biol. 11:1870-1873. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

